L-(-)-Xylose Chemische Eigenschaften,Einsatz,Produktion Methoden
R-Sätze Betriebsanweisung:
R36/37/38:Reizt die Augen, die Atmungsorgane und die Haut.
S-Sätze Betriebsanweisung:
S24/25:Berührung mit den Augen und der Haut vermeiden.
S36:DE: Bei der Arbeit geeignete Schutzkleidung tragen.
S26:Bei Berührung mit den Augen sofort gründlich mit Wasser abspülen und Arzt konsultieren.
Chemische Eigenschaften
L-Xylose is a white fine crystalline powder. It is an aldopentose that has a molecular formula C5H10O5 and a molar mass of 150.13 g/mol. The structure of L-xylose is presented in Figure 1. The optical rotations of D- and L-xylose are α20D = -18.6° and α20D = +18.6°, respectively (Fischer and Ruff, 1900). L-Xylose can exist in open chain form, as a pyranose or as a furanose. The relative concentrations of different stereoisomers have not been specified for L-xylose. However, for D-xylose the distribution of different forms in water are: 36.5% α-pyranose, 6% β-pyranose and less than 1 % in open chain and furanose form (Pastinen, 2000). Most likely L-xylose acts similarly to its enantiomer, and thus pyranose rings are predominant.
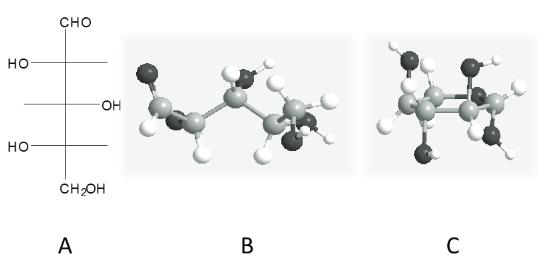
Figure 1. Structure of L-xylose A) as a Fischer projection, B) as a ball and stick model in acyclic form and C) as a ball and stick model in pyranose ring form.
Verwenden
L-Xylose is used in the synthesis of L-Xylose derivatives as selective sodium-dependent glucose cotransporter 2 (SGLT2) inhibitors for the treatment of type 2 diabetes.
Definition
ChEBI: Aldehydo-L-xylose is a xylose of ring-opened form having L-configuration.
Application
l-Xylose is a suitable starting material for the production of L-ribonucleosides. L- Xylose can be converted into an L-ribose derivative via oxidation and reduction steps. This product is then glycosylated to give L-ribonucleosides: L-uridine, L-cytidine, L- adenosine and L-guanosine (Moyroud and Strazewski, 1999; Chelain et al., 1995).
L-Xylose can also be used as a starting material for the production of polyhydroxypyrrolidines and related analogues. These compounds have many biological activities. They are shown to have anti-HIV effects, inhibit tumor growth, and also act as ?- and ?-glucosidase inhibitors, which is of relevance for the development of diabetes drugs (Behr and Guillerm, 2007).
synthetische
L-Xylose can be produced from L-xylulose by isomerization. This can be achieved either enzymatically or chemically under alkaline conditions. The equilibrium of the reaction between D-xylose and D-xylulose in an aqueous solution has been determined at pH 6.8-7.4 and at 25 °C to be 85:15 in favor of D-xylose (Tewari et al., 1985).
L-(-)-Xylose Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Downstream Produkte

