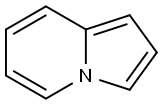
INDOLIZINE
- CAS No.
- 274-40-8
- Chemical Name:
- INDOLIZINE
- Synonyms
- Indozine;Pyrindole;Indolizin;INDOLIZINE;Pyrrocolin;Pyrrocoline;4-Azaindene;4-Azaindene Indolizine;Pyrrolo[1,2-a]pyridine;Pyrrolo[1,2-a]pyridine>
- CBNumber:
- CB2133649
- Molecular Formula:
- C8H7N
- Molecular Weight:
- 117.15
- MDL Number:
- MFCD00030189
- MOL File:
- 274-40-8.mol
- MSDS File:
- SDS
| Melting point | 75°C |
|---|---|
| Boiling point | 205.05°C |
| Density | 1.0224 (rough estimate) |
| refractive index | 1.5211 (estimate) |
| storage temp. | <0°C |
| CAS DataBase Reference | 274-40-8 |
| FDA UNII | B48FMH8YFQ |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H315-H319 |
| Precautionary statements | P264-P280-P302+P352+P332+P313+P362+P364-P305+P351+P338+P337+P313 |
INDOLIZINE price More Price(20)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | I627275 | Indolizine | 274-40-8 | 25mg | $165 | 2021-12-16 | Buy |
| Activate Scientific | AS146020 | Indolizine 95% | 274-40-8 | 250mg | $404 | 2021-12-16 | Buy |
| Activate Scientific | AS146020 | Indolizine 95% | 274-40-8 | 1g | $813 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | CHM0030127 | INDOLIZINE 95.00% | 274-40-8 | 1G | $1042.97 | 2021-12-16 | Buy |
| AK Scientific | 2610AB | Indolizine | 274-40-8 | 1g | $1091 | 2021-12-16 | Buy |
INDOLIZINE Chemical Properties,Uses,Production
Physical Properties
Indolizine and its alkyl derivatives are generally of low-mp or high- bp liquid, sensitive to light and air. They are steam volatile. The stability of indolizines is increased by the presence of substituents. The presence of aryl substituent at both the C2- and C5-positions increases stability and decreases steam volatility. The pKaH (3.9) of indolizine is much more basic than indole (pKaH −3.5).
Chemical Reactivity
Indolizines are chemically quite reactive and readily undergo electrophilic substitution reactions similar to indole and isoindoles but show resistance to nucleophilic substitution reactions. The preferential site for electrophilic substitution is C3 but may also take place at C1 as evident from the resonating structures. It is resistant to acid-catalyzed polymerization. As regards protonation of indolizine it is protonated preferentially at C3 but in the case of the preoccupied C3-position by an electron-donating substituent, protonation takes place at C1.
Uses
Indolizine is a useful chemical intermediate
Definition
ChEBI: Indolizine is a mancude organic heterobicyclic parent and a member of indolizines.
Synthesis Reference(s)
Journal of the American Chemical Society, 81, p. 1456, 1959 DOI: 10.1021/ja01515a044
The Journal of Organic Chemistry, 22, p. 589, 1957 DOI: 10.1021/jo01356a621
Purification Methods
Purify indolizine through an alumina column in *C6H6 and elute with *C6H6 (toluene could be used instead). The eluate contained in the fluorescent band (using UV light 365mn) is collected, evaporated and the crystalline residue is sublimed twice at 40-50o/0.2-0.5mm. The colourless crystals darken on standing and should be stored in dark sealed containers. If the original sample is dark in color then it should be covered with water and steam distilled. The colourless crystals in the distillate are collected and dried between filter paper and sublimed. It protonates on C3 in aqueous acid. It should give one fluorescent spot on paper chromatography (Whatman 1) in 3% aqueous ammonia and in n-BuOH/AcOH/H2O (4:1:1). The picrate has m 101o from EtOH. [Armarego J Chem Soc 226 1964, Armarego J Chem Soc (B) 191 1966, Scholtz Chem Ber 45 734 1912, Beilstein 20 II 200, 20 III/IV 3195.]
INDOLIZINE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Capot Chemical Co.,Ltd. | 571-85586718 +8613336195806 | sales@capotchem.com | China | 29797 | 60 |
| Alchem Pharmtech,Inc. | 8485655694 | sales@alchempharmtech.com | United States | 63711 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | 571-88938639 +8617705817739 | info@dycnchem.com | CHINA | 52867 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 9116 | 58 |
| Nantong HI-FUTURE Biology Co., Ltd. | +undefined18051384581 | sales@chemhifuture.com | China | 3136 | 58 |
| PT CHEM GROUP LIMITED | +86-85511178 +86-85511178 | peter68@ptchemgroup.com | China | 35453 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 | info@gihichemicals.com | China | 49999 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
Related articles
- Synthesis of Indolizine
- Indolizine is a bicyclic ring system compound, constituted by fusion of pyrrole and aryl rings in such a way that the nitrogen....
- Jan 24,2022
274-40-8(INDOLIZINE)Related Search:
1of4