ChemicalBook >> CAS DataBase List >>1-BOC-INDOLINE
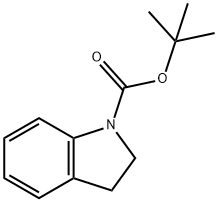
1-BOC-INDOLINE
- CAS No.
- 143262-10-6
- Chemical Name:
- 1-BOC-INDOLINE
- Synonyms
- 1-BOC-INDOLINE;BUTTPARK 52\06-71;1-(tert-Butoxycarbonyl)indoline;TERT-BUTYL INDOLINE-1-CARBOXYLATE;N-(tert-Butoxycarbonyl)-2,3-dihydroindole;tert-butyl 2,3-dihydroindole-1-carboxylate;2,3-dihydroindole-1-carboxylic acid tert-butyl ester;1H-Indole-1-carboxylic acid, 2,3-dihydro-, 1,1-diMethylethyl ester
- CBNumber:
- CB2169468
- Molecular Formula:
- C13H17NO2
- Molecular Weight:
- 219.28
- MDL Number:
- MFCD01318399
- MOL File:
- 143262-10-6.mol
Last updated:2023-07-14 17:31:15
| Melting point | 46-50 °C(lit.) |
|---|---|
| Boiling point | 83-84 °C0.1 mm Hg(lit.) |
| Density | 1.114±0.06 g/cm3(Predicted) |
| Flash point | >230 °F |
| pka | 0.84±0.20(Predicted) |
| BRN | 5336249 |
| CAS DataBase Reference | 143262-10-6 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| NFPA 704 |
|
1-BOC-INDOLINE price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 510149 | tert-Butyl indoline-1-carboxylate 98% | 143262-10-6 | 5g | $20.2 | 2023-06-20 | Buy |
| Alfa Aesar | L17477 | 1-Boc-indoline, 98% | 143262-10-6 | 5g | $40.6 | 2021-12-16 | Buy |
| Alfa Aesar | L17477 | 1-Boc-indoline, 98% | 143262-10-6 | 1g | $18.2 | 2021-12-16 | Buy |
| TRC | B663400 | 1-Boc-Indoline | 143262-10-6 | 10mg | $45 | 2021-12-16 | Buy |
| TRC | B663400 | 1-Boc-Indoline | 143262-10-6 | 50mg | $60 | 2021-12-16 | Buy |
1-BOC-INDOLINE Chemical Properties,Uses,Production
Uses
Reactant in preparation of aryl alkyl amines via alkylation reactions catalyzed by Pd 1 Reactant in preparation of allyl- and arylindolines 2 Reactant in modular indole synthesis of the highly strained CDEF parent tetracycle of nodulisporic acids A and B 3 Reactant in asymmetric synthesis of β-amino esters via rhodium prolinate complex-catalyzed α-C-H activation / carbenoid insertion reactions 4 Reactant in preparation of substituted indolines and tetrahydroquinolines via cycloaddition approach.
Uses
- Reactant in preparation of aryl alkyl amines via alkylation reactions catalyzed by Pd
- Reactant in preparation of allyl- and arylindolines
- Reactant in modular indole synthesis of the highly strained CDEF parent tetracycle of nodulisporic acids A and B
- Reactant in asymmetric synthesis of β-amino esters via rhodium prolinate complex-catalyzed α-C-H activation / carbenoid insertion reactions
- Reactant in preparation of substituted indolines and tetrahydroquinolines via cycloaddition approach
General Description
tert -Butyl indoline-1-carboxylate is an N-substituted indoline derivative.
1-BOC-INDOLINE Preparation Products And Raw materials
Global( 43)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Nanjing ChemLin Chemical Industry Co., Ltd. | 025-83697070 | product@chemlin.com.cn | CHINA | 3012 | 60 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32480 | 60 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| Sichuan Biosynce Pharmatech Co., Ltd. | +8619950309693 | diane@biosynce.com | China | 4874 | 58 |
| Aladdin Scientific | +1-833-552-7181 | sales@aladdinsci.com | United States | 57511 | 58 |
| Amadis Chemical Company Limited | 571-89925085 | sales@amadischem.com | China | 131981 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 4006608290; 18621169109 | market03@meryer.com | China | 40241 | 62 |
| Alfa Aesar | 400-6106006 | saleschina@alfa-asia.com | China | 30132 | 84 |
| Energy Chemical | 021-021-58432009 400-005-6266 | sales8178@energy-chemical.com | China | 44751 | 61 |
| Adamas Reagent, Ltd. | 400-6009262 16621234537 | zhangsn@titansci.com | China | 14113 | 59 |
143262-10-6(1-BOC-INDOLINE)Related Search:
INDOLE-3-ACETIC-ALPHA,ALPHA-D2 ACID
Indole-5-carboxaldehyde
Methyl indole-4-carboxylate
Methyl indole-6-carboxylate
DIMETHYL INDOLE-2,3-DICARBOXYLATE
1H-Indole-2-carboxamide,2,3-dihydro-(9CI)
Indolizine-2-carboxylic acid methyl ester
7-INDOLINECARBOXALDEHYDE
1-BOC-INDOLINE
6-AMINO-2,3-DIHYDRO-INDOLE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
1of4
BUTTPARK 52\06-71
1-BOC-INDOLINE
TERT-BUTYL INDOLINE-1-CARBOXYLATE
1H-Indole-1-carboxylic acid, 2,3-dihydro-, 1,1-diMethylethyl ester
N-(tert-Butoxycarbonyl)-2,3-dihydroindole
1-(tert-Butoxycarbonyl)indoline
2,3-dihydroindole-1-carboxylic acid tert-butyl ester
tert-butyl 2,3-dihydroindole-1-carboxylate
143262-10-6
Heterocyclic Building Blocks
Indoles
Building Blocks
Building Blocks
Heterocyclic Building Blocks
Indoles
Heterocyclic Compounds