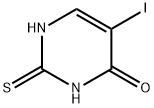
Iodothiouracil
- CAS No.
- 5984-97-4
- Chemical Name:
- Iodothiouracil
- Synonyms
- Itrumil;Iothiouracil;Iodothiouracil;Jodthiouracilum;5-Iodothiouracil;5-Iodo-2-thiouracil;5-Iodo-2-mercaptopyrimidin-4-ol;5-iodo-2-sulfanylidene-1H-pyrimidin-4-one;5-Iodo-2-thioxo-2,3-dihydropyrimidin-4(1H);2-Mercapto-5-iodo-1,4-dihydropyrimidine-4-one
- CBNumber:
- CB31178961
- Molecular Formula:
- C4H3IN2OS
- Molecular Weight:
- 254.05
- MDL Number:
- MFCD00869151
- MOL File:
- 5984-97-4.mol
| Density | 2.36±0.1 g/cm3(Predicted) |
|---|---|
| Melting point | 278-280 °C (decomp) |
| pka | 6.48±0.25(Predicted) |
| FDA UNII | 61O17612T5 |
| EPA Substance Registry System | 5-Iodo-2-thiouracil (5984-97-4) |
Iodothiouracil price More Price(5)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Matrix Scientific | 099508 | 5-Iodo-2-thioxo-2,3-dihydropyrimidin-4(1H)-one 95+% | 5984-97-4 | 1g | $378 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | HCH0125999 | 2,3-DIHYDRO-5-IODO-2-THIOXO-4(1H)-PYRIMIDINONE 95.00% | 5984-97-4 | 5MG | $498.91 | 2021-12-16 | Buy |
| Matrix Scientific | 099508 | 5-Iodo-2-thioxo-2,3-dihydropyrimidin-4(1H)-one 95+% | 5984-97-4 | 5g | $1008 | 2021-12-16 | Buy |
| AK Scientific | 8915AB | 5-Iodo-2-thioxo-2,3-dihydropyrimidin-4(1H)-one | 5984-97-4 | 5g | $1410 | 2021-12-16 | Buy |
| Crysdot | CD11097093 | 5-Iodo-2-thioxo-2,3-dihydropyrimidin-4(1H)-one 95+% | 5984-97-4 | 5g | $480 | 2021-12-16 | Buy |
Iodothiouracil Chemical Properties,Uses,Production
Originator
Itrumil,Ciba,US,1951
Definition
ChEBI: Iodothiouracil is an organohalogen compound and a member of pyrimidines.
Manufacturing Process
As an illustrative example 64.4 g of 5-iodo-2-benzyl thiouracil were deposited
in the reaction vessel and dissolved by adding 400 cc of glacial acetic acid
containing 10 cc of acetic anhydride and the reaction vessel was connected
tightly with the reflux condenser. The second vessel or generator was charged
with 95 cc of acetic anhydride and the vessel connected to a vessel such as a
dropping funnel or equivalent containing 75 cc of a 50% solution of hydroiodic
acid which was added slowly, as by dropwise addition, to the acetic anhydride
in the generator. The mixture in the generator soon became hot and the
hydrogen iodide which evolved passed continuously through the connecting
conduit into the reaction flask just above the level of liquid therein. As the
hydrogen iodide contacted the solution of the 2 benzyl derivative,a ring of the
debenzylated product formed under the inlet conduct. This operation was
continued until all of the hydroidic acid was added to the generator vessel.
The hydrogen iodide remaining in the generator was driven over into the
reaction vessel by heating the generator. It was ascertained that the reaction
is complete when no more precipitate forms in the main reaction vessel.
During the reaction vapors evolved were condensed in the condenser and
returned to the reaction vessel as reflux. The upper end of the reflux ispreferably connected with a vent leading to a drying chamber.
The reaction vessel was cooled and the precipitate separated by pouring or
decanting off the supernatant liquor. The precipitate of the 5-iodo-2-thiouracil
was then thoroughly washed, as, for example, on a Buchner funnel. The
precipitate was then extracted twice with hot glacial acetic acid to remove
unreacted material and then washed thoroughly by alternate washes with
alcohol and water. The product was then further purified by dissolving it in
warm dilute sodium hydroxide and after cooling was reprecipitated by careful
acidulation with acetic acid. Utilizing this procedure 37 g of purified 5-iodo-2-
thiouracil were obtained.
The supernatant liquid separated from the precipitate was concentrated in
vacuo and 7.4 g of the unreacted 5-iodo-2-benzyl thiouracil were recovered.
This obviously may be utilized for further debenzylation.
As pointed out previously, the 5-iodo-2-thiouracil is carefully dried, preferably
in a vacuum over P2O5.
Therapeutic Function
Thyroid inhibitor
Iodothiouracil Preparation Products And Raw materials
Iodothiouracil Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Jilin Chinese Academy of Sciences-yanshen Technology | +undefined18143011203 | info@chemextension.com | China | 42057 | 58 |
| Shanghai Hekang Biotechnology Co., Ltd. | 18939837085 | youchemicals@gmail.com | China | 1813 | 55 |
| BePharm Ltd | 400-685-9117 | market@bepharm.com | China | 15081 | 60 |
| Aikon International Limited | 025-58851090 13611564524 | lwan@aikonchem.com | China | 15952 | 58 |
| Ningbo jinuo chemical co., LTD | 0574-87314919 057487319282 | info@nbinno.com | China | 7216 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Shanghai Haohong Scientific Co., Ltd. | 400-400-8210725 | malulu@leyan.com | China | 40041 | 58 |
| Jilin Chinese Academy of Sciences-yanshen Technology | 18143011203 | ET-market@chemextension.com | China | 30422 | 58 |