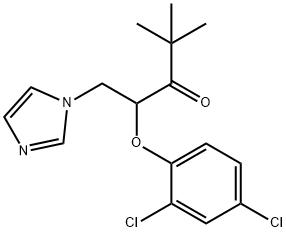
Valconazole
- CAS No.
- 56097-80-4
- Chemical Name:
- Valconazole
- Synonyms
- Valconazole;3-Pentanone, 2-(2,4-dichlorophenoxy)-1-(1H-imidazol-1-yl)-4,4-dimethyl-
- CBNumber:
- CB31180034
- Molecular Formula:
- C16H18Cl2N2O2
- Molecular Weight:
- 341.23
- MDL Number:
- MFCD00866230
- MOL File:
- 56097-80-4.mol
| Boiling point | 501.1±50.0 °C(Predicted) |
|---|---|
| Density | 1.24±0.1 g/cm3(Predicted) |
| pka | 6.41±0.12(Predicted) |
| FDA UNII | 3EVI8KD2BC |
Valconazole Chemical Properties,Uses,Production
Originator
Valconazole,JINGTIAN PORTLINK CO., LTD
Manufacturing Process
1). 0.9 moles 4-(2,4-dichlorophenoxy)-2,2-dimethylbutan-3-one, 1 mole
trimethyl ammonium chloride and 1 mole paraformaldehyde were in 300 ml
dry ethanol dissolved. 2 ml conc. hydrochloric acid was added and the mixture
was heated to reflux for 2 hours. After adding of 1 extra mole of
paraformaldehyde the mixture was heated to reflux for 2 hours and stood for night at room temperature. Then it was poured into 1.2 L water and extracted
with 1.5 L ether. The water layer was basified with solution of ammonia to pH
8 and extracted with 1 L ether. The ether phase was separeted and dried over
sodium sulphate and the solvent was removed in vacuum to give [2-(2,4-
dichlorophenoxy)-4,4-dimethyl-3-oxopentyl]trimethylammonium chloride.
2). 0.1 mol of above ammonium chloride was dissolved in 200 ml of dry
tetrahydrofuran and 0.2 moles triethylamine was added by stirring at room
temperature. A precipitate of triethylammonium chloride was filtered off and
filtrate was distilled to dryness. The residue was immediately dissolved in 300
ml dry acetonitrile and 0.15 moles of methyl iodide were added dropwise at
room temperature and by stirring. The mixture was stood at room
temperature for 1 hour. Then it boiled for 30 minutes. After that, the solvents
were evaporated and the residue was heated to boiling in the mixture of ethyl
acetate/methyl ethyl ketone (1:1). On cooling the precipitate of [2-(2,4-
dichlorophenoxy)-4,4-dimethyl-3-oxopentyl]trimethylammonium iodide was
filtered off and washed with ether.
3). To a solution of 0.0464 moles [2-(2,4-dichlorophenoxy)-4,4-dimethyl-3-
oxopentyl]trimethylammonium iodide in 250 ml dry acetonitrile 0.15 moles of
imidazole was added and heated to reflux for 24 hours. The solvent was
removed in vacuum. The residue was dissolved in 500 ml methylene chloride
and washed with water. The organic phase was separated and dried over
sodium sulphate. Methylene chloride was distilled off to give 2-(2,4-
dichlorophenoxy)-1-(1H-imidazol-1-yl)-4,4-dimethyl-3-pentanone melted at
85-87°C. Hydrochloride melted at 118°C.
Therapeutic Function
Antifungal
Valconazole Preparation Products And Raw materials
Raw materials
Preparation Products
Valconazole Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 | support@targetmol.com | United States | 19973 | 58 |
| Supplier | Advantage |
|---|---|
| Hangzhou MolCore BioPharmatech Co.,Ltd. | 58 |
| TargetMol Chemicals Inc. | 58 |