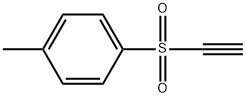
ETHYNYL P-TOLYL SULFONE
- CAS No.
- 13894-21-8
- Chemical Name:
- ETHYNYL P-TOLYL SULFONE
- Synonyms
- 1-(ethynylsulfonyl)-4-Methylbenzene;TOSYLACETYLENE;ETHYNYL P-TOLYL SULFONE;Ethynylp-TolylSulfone>p-tolylsulfonylacetylene;4-(Ethynylsulfonyl)toluene;P-TOLUENESULFONYLACETYLENE;Ethynyl p-Tolyl Sulfone;Benzene, 1-(ethynylsulfonyl)-4-methyl-;p-Toluenesulfonylacetylene, Tosylacetylene
- CBNumber:
- CB3427572
- Molecular Formula:
- C9H8O2S
- Molecular Weight:
- 180.22
- MDL Number:
- MFCD00191647
- MOL File:
- 13894-21-8.mol
- MSDS File:
- SDS
| Melting point | 73-74 °C (lit.) |
|---|---|
| Boiling point | 297.9±33.0 °C(Predicted) |
| Density | 1.219±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | organic solvents: soluble(lit.) |
| form | powder to crystal |
| color | White to Light yellow |
| BRN | 2556169 |
| UNSPSC Code | 12352100 |
| NACRES | NA.22 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H315-H319-H335 | |||||||||
| Precautionary statements | P261-P264-P271-P280-P302+P352-P305+P351+P338 | |||||||||
| Hazard Codes | Xi | |||||||||
| Risk Statements | 36/37/38 | |||||||||
| Safety Statements | 26-36 | |||||||||
| WGK Germany | 3 | |||||||||
| HS Code | 29041000 | |||||||||
| NFPA 704 |
|
ETHYNYL P-TOLYL SULFONE price More Price(15)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 419869 | Ethynyl p-tolyl sulfone 98% | 13894-21-8 | 1g | $113 | 2025-07-31 | Buy |
| TCI Chemical | E0466 | Ethynyl p-Tolyl Sulfone >98.0%(GC) | 13894-21-8 | 1g | $201 | 2025-07-31 | Buy |
| TCI Chemical | E0466 | Ethynyl p-Tolyl Sulfone >98.0%(GC) | 13894-21-8 | 5g | $638 | 2025-07-31 | Buy |
| TRC | E938863 | Ethynyl p-Tolyl Sulfone | 13894-21-8 | 50mg | $45 | 2021-12-16 | Buy |
| AK Scientific | 7929AL | Ethynyl p-Tolyl Sulfone | 13894-21-8 | 5g | $343 | 2021-12-16 | Buy |
ETHYNYL P-TOLYL SULFONE Chemical Properties,Uses,Production
Uses
Ethynyl p-tolyl sulfone (Tosylacetylene) may be used in the synthesis of 2-(4-methylphenylsulfonyl)ethenyl (tosvinyl, Tsv) protected derivatives. It may be used as starting reagent for the synthesis of optically active indan-2-ols.
Application
Ethynyl p-Tolyl Sulfone is a dienophile used in electrocyclic reactions such as dipolar cycloaddition, Diels-Alder reaction, and ene cyclization; electron-poor Michael acceptor in conjugate additions; undergoes direct displacement with organometallic complexes to give aryl- or alkylacetylenes.
Preparation
Ethynyl p-Tolyl Sulfone has been prepared by several groups over the years employing a variety of synthetic
strategies including ethynyl Grignard addition to p-toluenesulfonyl fluoride, dehydroiodination of (E)-2-iodo-1-
tosyl-1-ethene, oxidative elimination of β-[(4-methylphenyl)seleno]vinyl sulfone, dehydrobromination of cis-and trans-2-bromovinyl 4-methylphenyl sulfone,23 diazotization of 4-[(4-methylphenyl)sulfonyl]-5-
aminoisoxazole, and oxidation of ethynyl thioether. The modified strategy commonly used today is an
established Friedel-Crafts based procedure first performed by Bhattacharya et al. but recently exploited by
Waykole and Paquette (eq 1). Its simplicity and mildness has shown it to be broadly useful since it bypasses
the need for immoderate conditions encountered in the earlier strategies.
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 697, 1992 DOI: 10.1021/jo00028a053
storage
Decomposition of the intermediate complex p-toluenesulfonyl chloride-aluminum chloride-bis(trimethylsilyl)acetylene with hydrochloric acid and ice is exothermic and due caution is recommended. Also, a nitrogen atmosphere is suggested throughout its preparation due to the hygroscopic nature of the reagents and intermediate(s) involved.
Purification Methods
Recrystallise the sulfone from pet ether, *C6H6 or EtOH (m 66o), and dry it in a vacuum. [Beilstein 6 III 1397, 6 IV 2160.]
ETHYNYL P-TOLYL SULFONE Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Frapp's ChemicalNFTZ Co., Ltd. | +86 (576) 8169-6106 | sales@frappschem.com | China | 880 | 50 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 33024 | 60 |
| Jinan Carbotang Biotech Co.,Ltd. | +8615866703830 | figo.gao@foxmail.com | China | 8497 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49733 | 58 |
| Shanghai Safer Pharmaceutical Technology Co., Ltd. | 021-31761238 | sales@saferph.com | CHINA | 897 | 58 |
| Neostar United (Changzhou) Industrial Co., Ltd. | +86-0519-85551759 +8613506123987 | marketing1@neostarunited.com | China | 8829 | 58 |
| Dayang Chem (Hangzhou) Co.,Ltd. | +86-0571-88938639 +8617705817739 | info@dycnchem.com | China | 52846 | 58 |
| CD Chemical Group Limited | +8615986615575 | info@codchem.com | China | 20342 | 58 |
| GLR Innovations | +91 9891111994 | info@glrgroup.in | India | 4535 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +8615531151365 | mina@chuanghaibio.com | China | 18126 | 58 |