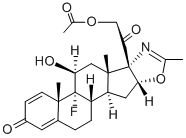
fluazacort
- CAS No.
- 19888-56-3
- Chemical Name:
- fluazacort
- Synonyms
- L-6400;Azacortid;fluazacort;Fluazacortum;21-acetoxy-9-fluoro-11β-hydroxy-2'-methyl-(16β)-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione;9α-Fluoro-11β,21-dihydroxy-3,20-dioxo-1,4-pregnadieno[17α,16α-d]-2-Methyloxazoline 21-Acetate;(16β)-21-(Acetoxy)-9-fluoro-11β-hydroxy-2'-methylpregnano[17,16-d]oxazole-1,4-diene-3,20-dione;(5'β)-9-Fluoro-11β,21-dihydroxy-2'-methylpregnano[17,16-d]oxazole-1,4-diene-3,20-dione 21-acetate;(11β,16β)-21-(Acetyloxy)-9-fluoro-11-hydroxy-2'-Methyl-5'H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione;5'H-Pregna-1,4-dieno[17,16-d]oxazole-3,20-dione, 21-(acetyloxy)-9-fluoro-11-hydroxy-2'-methyl-, (11β,16β)-
- CBNumber:
- CB3909984
- Molecular Formula:
- C25H30FNO6
- Molecular Weight:
- 459.51
- MDL Number:
- MOL File:
- 19888-56-3.mol
| Melting point | 252-255° |
|---|---|
| alpha | D +54.8° (c = 0.5 in CHCl3) |
| FDA UNII | Y37GZ3VFSS |
fluazacort price More Price(4)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | F407400 | Fluazacort | 19888-56-3 | 10mg | $195 | 2021-12-16 | Buy |
| TRC | F407400 | Fluazacort | 19888-56-3 | 100mg | $1540 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0011466 | FLUAZACORT 95.00% | 19888-56-3 | 100MG | $2194.5 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0011466 | FLUAZACORT 95.00% | 19888-56-3 | 10MG | $779.63 | 2021-12-16 | Buy |
fluazacort Chemical Properties,Uses,Production
Originator
Azacortid,Richter,Italy,1975
Uses
fluazacort is a glucucorticoid with anti-inflammatory properties used in the treatment of skin diseases.
Definition
ChEBI: Fluazacort is a corticosteroid hormone.
Manufacturing Process
To a solution of 2.4 g of pregna-1,4,9(11)-triene-21-ol-3,20-dione-[17α,16α-
d]-2'-methyloxazoline 21-acetate in 24 ml of tetrahydrofuran, 12.8 ml of 0.46
N perchloric acid are added at 15°C under stirring. N-bromoacetamide (1.1 g)
is then added to the mixture which is kept far from light, and stirred for 4
hours at room temperature. After lowering the temperature to 10°C, a
saturated solution of sodium bisulfite is added in order to decolorize the
mixture, which is then poured into 120 ml of ice water. A product separates,
which is collected by filtration, washed with water and then dried, thus
obtaining 2.81 g of crude 9α-bromo-pregna-1,4-diene-11β,21-diol-3,20-dione-
[17α,16α-d]-2'-methyloxazoline-21-acetate (yield 93%), MP 175°C to 176°C.
An amount of 2.75 g of 9α-brorno-pregna-1,4-diene-11β,21-diol-3,20-dione-
[17α,16α-d]-2'-methyloxazoline-21-acetate is dissolved under nitrogen in 137
ml of a mixture methanol:chloroform (3:2). The solution is put in ice bath and
5.5 ml of 1 N NaOH are then added within 10 minutes followed by 5.5 ml
within the next 40 minutes. A strong stirring is provided for 2 hours and the
temperature is kept between 0°C and 5°C, then the pH is adjusted to 7 to 8
with glacial acetic acid. The solvent is evaporated in vacuo to 20 ml of volume
of solution, that is poured into ice water (130 ml). The product is collected by
filtration, washed with water and dried. Yield: 1.6 g (80%), MP 221°C to
222°C. It is pregna-1,4-diene-9β,11β-epoxy-21-ol-3,20-dione-[17α,16α-d]-2'-
methyloxazoline.
An amount of 1 g of the above product is dissolved in 9.4 ml of a mixture
obtained by mixing 4.67 ml of hydrofluoric acid with 8.5 ml of tetrahydrofuran
at the temperature of 0°C. This solution is stirred for 20 hours at the same
temperature, then under strong stirring and cooling 20 ml of tetrahydrofuran
are added. The solution is subsequently neutralized by the addition of 24 g of
sodium bicarbonate followed by 1 g of sodium sulfate. The inorganic
substance is collected and washed with ethyl acetate. The filtrate is
evaporated to dryness and the product is crystallized from acetone: 0.65 g
(yield 61%) of pregna-1,4-dien-9α-fluoro-11β,21-diol-3,20-dione-[17α,16α-d]-
2'-methyloxazoline are obtained, MP 241°C to 244°C [α]D = +83.5 (c. 0.5,
CHCl3).The 21-acetate has MP 252°C to 255°C [α]D = +54.8 (c. 0.5, CHCl3).
Therapeutic Function
Antiinflammatory
fluazacort Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Hangzhou MolCore BioPharmatech Co.,Ltd. | +86-057181025280; +8617767106207 | sales@molcore.com | China | 49739 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| Shenzhen Polymeri Biochemical Technology Co., Ltd. | +86-400-002-6226 13028896684 | sales@rrkchem.com | China | 55827 | 58 |
| Energy Chemical | 021-58432009 400-005-6266 | marketing@energy-chemical.com | China | 44941 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |