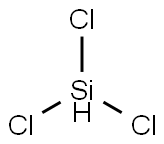
Trichlorosilane
- CAS No.
- 10025-78-2
- Chemical Name:
- Trichlorosilane
- Synonyms
- trichlorsilan;silicon chloroform;orosiL;TrichL;silanea-19;SiHCl3(TCS) ;triclorosilano;Trichorosilane;TRICHLOROSILANE;trichloorsilaan
- CBNumber:
- CB4852559
- Molecular Formula:
- Cl3HSi
Lewis structure
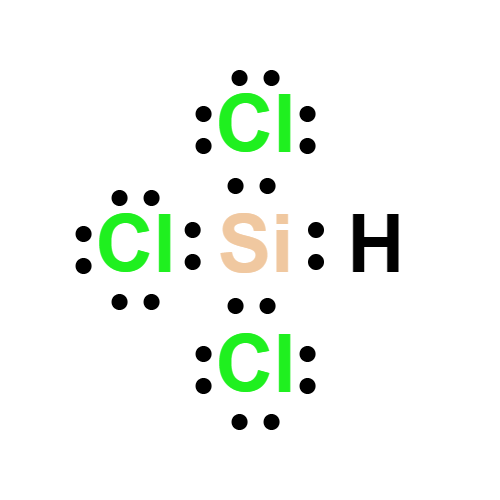
- Molecular Weight:
- 135.45
- MDL Number:
- MFCD00011518
- MOL File:
- 10025-78-2.mol
- MSDS File:
- SDS
| Melting point | -127 °C |
|---|---|
| Boiling point | 32-34 °C(lit.) |
| Density | 1.342 g/mL at 25 °C(lit.) |
| vapor density | 1 (vs air) |
| vapor pressure | 9.75 psi ( 20 °C) |
| refractive index | 1.4-1.402 |
| Flash point | 7 °F |
| storage temp. | Refrigerator |
| solubility | Soluble in benzene, ether, heptane, chloroform and carbon tetrachloride. |
| form | Liquid |
| Specific Gravity | 1.342 |
| color | Colorless |
| Odor | Sharp, choking, like hydrochloric acid. |
| explosive limit | 70% |
| Water Solubility | decomposes |
| Sensitive | Air & Moisture Sensitive |
| Hydrolytic Sensitivity | 8: reacts rapidly with moisture, water, protic solvents |
| Merck | 14,9646 |
| Stability | Stable, but extremely flammable. Pyrophoric - spontaneously ignites in air. Explosion hazard in air - note wide explosion limits. Reacts violently with water. Incompatible with moisture, acids, bases, strong oxidizing agents, alcohols, amines. |
| InChIKey | ZDHXKXAHOVTTAH-UHFFFAOYSA-N |
| CAS DataBase Reference | 10025-78-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| FDA UNII | QZY2645L6V |
| NIST Chemistry Reference | Trichlorosilane(10025-78-2) |
| EPA Substance Registry System | Trichlorosilane (10025-78-2) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS02,GHS05,GHS06 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H224-H250-H302-H314-H331-H335 | |||||||||
| Precautionary statements | P210-P233-P280-P303+P361+P353-P305+P351+P338-P403+P233 | |||||||||
| Hazard Codes | F+,C | |||||||||
| Risk Statements | 12-14-17-20/22-29-35 | |||||||||
| Safety Statements | 16-26-36/37/39-43-45-7/9-43A | |||||||||
| RIDADR | UN 1295 4.3/PG 1 | |||||||||
| WGK Germany | 1 | |||||||||
| RTECS | VV5950000 | |||||||||
| F | 4.5-21-31 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 4.3 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 28530090 | |||||||||
| Toxicity | LD50 orally in rats: 1.03 g/kg (Smyth) | |||||||||
| NFPA 704 |
|
Trichlorosilane price More Price(8)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 175552 | Trichlorosilane 99% | 10025-78-2 | 5g | $52.9 | 2024-03-01 | Buy |
| Sigma-Aldrich | 175552 | Trichlorosilane 99% | 10025-78-2 | 100g | $61.2 | 2024-03-01 | Buy |
| Alfa Aesar | 014078 | Trichlorosilane 98% | 10025-78-2 | 25g | $26.5 | 2023-06-20 | Buy |
| Alfa Aesar | 014078 | Trichlorosilane 98% | 10025-78-2 | 100g | $64.3 | 2023-06-20 | Buy |
| Sigma-Aldrich | 175552 | Trichlorosilane 99% | 10025-78-2 | 500g | $159 | 2024-03-01 | Buy |
Trichlorosilane Chemical Properties,Uses,Production
Physical and chemical properties
Trichlorosilane is pungent stench flowable volatile colorless liquid at room temperature and pressure. It is highly flammable in air, it also has the risk of fire at below-18 ℃, when meets open fire, it burns strongly with red flames and white smoke, and SiO2, HCl and Cl2 can generate: SiHCl3 + O2 → SiO2 + HCl + Cl2; the vapor of three chlorosilane and air can form a wide range of concentrations of explosive gas mixture, and it can cause violent explosion when be heated. The thermal stability is better than dichlorosilane, it can decompose into toxic fumes chloride (HCl), Cl2 and Si at 900℃. When meets moisture, it can generate smoke, it can react violently with water: 2SiHCl3 + 3H2O → (HSiO) 2O + 6HCl; It can decompose in lye and release hydrogen: SiHCl3 + 3NaOH + H2O → Si (OH) 4 + 3NaCl + H2; and when it contacts oxidative substances, explosive reaction can occur. The reaction with acetylene, hydrocarbons and other organic hydrocarbons can produce chlorosilane: SiHCl3 + CH≡CH → CH2CHSiCl3, SiHCl3 + CH2 = CH2 → CH3CH2SiCl3; In the presence of lithium aluminum hydride, lithium borohydride, SiHCl3 can be reduced to silane. When the container is subjected to strong shock, liquid SiHCl3 will fire. It is soluble in benzene, ether etc.. Anhydrous trichlorosilane can not corrode iron and stainless steel, but in the presence of moisture, it can corrode most metals.
Trichlorosilane
Trichlorosilane is also known as trichlorosilane, silicon chloroform, it can be used the raw material of polymer organic silicon compound.
Trichlorosilane is not only the important intermediate of manufacturing silane coupling agent, and other organic silicon products, but also the major raw material to manufacture polysilicon. Silane coupling agent is an important, high-tech, high value-added silicone composite material, the non-crosslinked resin can crosslink or modify by silane coupling agent, so it is increasingly and widely used in glass fiber, foundry, tires rubber and other industries. Silicon tetrachloride which is the major byproduct in the production of trichlorosilane is also the main raw material of silicone, the manufactures contain silicon ester, silicone oil, high temperature insulation paint, silicone resin, silicone rubber and heat-resistant padded material . High-purity silicon tetrachloride or high-purity silica is important raw material in manufacturing of inorganic silicon compounds, quartz fibers and optical fiber.
The main purpose of the polysilicon product:
1. It can be made into solar cell, the radiant energy can be turned into electrical energy; 2. high purity crystalline silicon is an important semiconductor material; 3. it is important material in cermet, Astronautical; 4. it can be used in optical fiber communication which is recent modern communication means; 5. it can be used in excellent properties of the organosilicon compounds.
The above information is edited by the chemicalbook of Wang Xiaodong.
Toxicity
After several minutes of inhaling trichlorosilane vapor, people show excitement, breathing reflex disorders, respiratory inflammation. When spills on the skin, it can cause necrosis, long-term healing of ulcers. Maximum allowable concentration is 1 mg/m3 (must test HCl). Production staff should wear closed-type overalls, protective masks, sealed glasses, latex gloves and other protective clothing to prevent direct contact with the respiratory organs, eyes and skin. Production equipment must be sealed, workshop should be ventilated. After work, people should take a shower, and aobserve personal hygiene. Production workers should have pre-employment and periodic medical examinations.
Chemical properties
It is colorless liquid. It is soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene etc.
Uses
It can be used the raw material for polymer organic silicon compound, it can also be used in industrial instrumentation.
It can be used the raw material for producing organic silicon compound, it is also the basic raw material of production polysilicon.
Production method
The method of boiling chlorination
Silicon powder after dried is added into boiling chloride furnace, and reacts with dry hydrogen chloride gas which passes into the furnace of at 340 ℃. The generated crude trichlorosilane goes through the wet dust collector, condenser tube and comes into distillation column to separate silicon tetrachloride, trichlorosilane gas from the distillation column is condensed to obtain finished trichlorosilane.
Si + 3HCl → SiHCl3 + H2
Category
Flammable liquid.
Toxicity grading
Mid toxicity.
Acute oral toxicity
rat LD50: 1030 mg/kg; Inhalation-Mouse LC50: 1500 mg/m/2 hours.
Flammability hazard characteristics
It is flammable in case of fire, high temperature, oxidant; it can generate toxic fumes of chlorides and fluorides in case of water or high temperature.
Storage characteristics
Treasury should be ventilated and low-temperature dried; it should be stored separately with oxidants, acids.
Extinguishing agent
Dry powder, dry sand, carbon dioxide, foam.
Chemical Properties
Clear liquid with acrid odor of hydrogen chloride. soluble in carbon disulfide, carbon tetrachloride, chloroform, benzene, etc.
Uses
Trichlorosilane is used in process of hydrosilylation. It is also used for reductive hydrazination which is a high yielding method for preparation of 1,1-disubstituted hydrazines.
Uses
In organic synthesis.
General Description
A colorless fuming liquid with a pungent odor. Flash point 7°F. Vapor and liquid cause burns. More dense than water. Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Ignites spontaneously in air [NFPA, 1991]. Reacts violently with water, steam, moisture in air to generate heat and flammable (H2) and corrosive (HCl) gases. [Handling Chemicals Safely 1980. p. 924].
Reactivity Profile
Trichlorosilane reacts with alcohols, acetone, light metals with generation of heat and combustible (H2) and corrosive (HCl) gases [Handling Chemicals Safely 1980. p. 924].
Health Hazard
Inhalation causes severe irritation of respiratory system. Liquid causes severe burns of eyes and skin. Ingestion causes severe burns of mouth and stomach.
Flammability and Explosibility
Substances and mixtures which in contact with water emit flammable gases
Safety Profile
Moderately toxic by ingestion and inhalation. A corrosive irritant to skin, eyes, and mucous membranes. A very dangerous fire hazard when exposed to heat, flame, or by chemical reaction. May be ignited by spark or impact. Spontaneously flammable in air. Explosive reaction with acetonitrile + diphenyl sulfoxide. Will react with water or steam to produce heat and toxic and corrosive fumes. Can react vigorously with oxidizing materials. To fight fire, use CO2, dry chemical. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES.
Trichlorosilane Preparation Products And Raw materials
Raw materials
Preparation Products
1of4
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hebei Mojin Biotechnology Co., Ltd | +86 13288715578 +8613288715578 | sales@hbmojin.com | China | 12446 | 58 |
| Shanghai Daken Advanced Materials Co.,Ltd | +86-371-66670886 | info@dakenam.com | China | 18628 | 58 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Shanghai Time Chemicals CO., Ltd. | +86-021-57951555 +8617317452075 | jack.li@time-chemicals.com | China | 1807 | 55 |
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 | ivan@atkchemical.com | China | 32836 | 60 |
| career henan chemical co | +86-0371-86658258 +8613203830695 | sales@coreychem.com | China | 29888 | 58 |
| Win-Win Chemical CO., Limited | 0086-577-64498589 | sales@win-winchemical.com | CHINA | 998 | 58 |
| Hebei Guanlang Biotechnology Co., Ltd. | +86-19930503282 | alice@crovellbio.com | China | 8820 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-592-6051114 +8618959220845 | sales@amoychem.com | China | 6387 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
View Lastest Price from Trichlorosilane manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-09-20 | Trichlorosilane
10025-78-2
|
US $7.98-6.93 / kg | 1kg | 98.86% | 18000 tons per year | Iota silicone oil (Anhui) Co.,ltd. | |
 |
2023-06-25 | Trichlorosilane
10025-78-2
|
US $0.00 / KG | 1KG | 99% | 50000KG/month | Hebei Mojin Biotechnology Co., Ltd | |
 |
2022-08-20 | Trichlorosilane
10025-78-2
|
US $10.00-5.00 / KG | 1KG | 99% | 10 mt | Hebei Guanlang Biotechnology Co., Ltd. |
-

- Trichlorosilane
10025-78-2
- US $7.98-6.93 / kg
- 98.86%
- Iota silicone oil (Anhui) Co.,ltd.
-

- Trichlorosilane
10025-78-2
- US $0.00 / KG
- 99%
- Hebei Mojin Biotechnology Co., Ltd
-

- Trichlorosilane
10025-78-2
- US $10.00-5.00 / KG
- 99%
- Hebei Guanlang Biotechnology Co., Ltd.
10025-78-2(Trichlorosilane)Related Search:
1of4