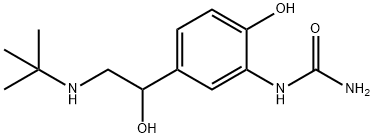
carbuterol
- CAS No.
- 34866-47-2
- Chemical Name:
- carbuterol
- Synonyms
- carbuterol;Carbuterolum;Unii-0N12jr32mr;Einecs 252-257-5;Carbuterolum [inn-latin];34866-46-1 (Mono-hydrochloride);[5-[2-(tert-Butylamino)-1-hydroxyethyl]-2-hydroxyphenyl]urea;Urea, N-[5-[2-[(1,1-dimethylethyl)amino]-1-hydroxyethyl]-2-hydroxyphenyl]-
- CBNumber:
- CB4917546
- Molecular Formula:
- C13H21N3O3
- Molecular Weight:
- 267.32
- MDL Number:
- MOL File:
- 34866-47-2.mol
| Melting point | 174-176° |
|---|---|
| solubility | DMSO (Slightly), Methanol (Slightly), Water (Slightly) |
| form | Solid |
| color | White to Pale Beige |
| Stability | Hygroscopic |
| FDA UNII | 0N12JR32MR |
| ATC code | R03AC10,R03CC10 |
SAFETY
Risk and Safety Statements
| Toxicity | LD50 in mice (mg/kg): 32.8 i.v.; 3134.6 orally; in rats: 77.2 i.v. (Wardell) |
|---|
carbuterol Chemical Properties,Uses,Production
Originator
Bronsecur,SK and F,W. Germany,1980
Uses
Carbuterol is a beta-adrenoceptor binding drug.
Uses
Bronchodilator.
Definition
ChEBI: Carbuterol is a member of ureas.
Manufacturing Process
A stirred solution of 40 g (0.41 m) of phosgene in 150 ml of toluene is held at
25°C with a cooling bath while a mixture of 252 g (0.105 m) of 3-amino-4-
benzyloxyacetophenone and 220 ml of toluene are added slowly. The mixture
is heated to reflux and continued for 30 minutes. Nitrogen is passed through
the mixture and then concentrated in vacuo to give a crystalline isocyanate,
MP 105°-106°C.
A stirred solution of the isocyanate (28.0 g) in 500 ml of dry benzene is
saturated with ammonia. After one hour, the mixture is cooled to give the
crystalline 4-benzyloxy-3-ureidoacetophenone, MP 184°-186°C.
To a stirred solution of 5.7 g (0.02 m) of 4-benzyloxy-2-ureidoacetophenone
in 100 ml of chloroform is added 32 g (0.02 m) of bromine. The mixture is
stirred at room temperature for about 45 minutes and the solution is
concentrated in vacuo at 25°-30°C. The amorphous residue (hydrobromide
salt of 4-benzyloxy-α-bromo-3-ureidoacetophenone) is dissolved in 80 ml of
acetonitrile and 98 g (0.06 m) of N-benzyl-N-t-butylamine is added. The
mixture is stirred and refluxed for 1.5 hours, then it is cooled to 0°C in an ice
bath. Crystalline N-benzyl-N-t-butylamine hydrobromide is filtered. The filtrate
is acidified with ethereal hydrogen chloride. The semicrystalline product is
filtered after diluting the mixture with a large excess of ether. Trituration of
the product with 60 ml of cold ethanol gives 4-benzyloxy-α-(N-benzyl-N-t_x0002_butylamino)-3-ureidoacetophenone hydrochloride, MP 200°-221°C
(decomposition).
A solution of 10.5 g (0.0218 m) of 4-benzyloxy-α-(N-benzyl-N-t-butylamino)-3-ureidoacetophenone hydrochloride in 65 ml of methanol and 25 ml of water
is added to a suspension of 1.5 g of 10% palladium-on-carbon in 10 ml of
water. The mixture is hydrogenated on the Parr apparatus at room
temperature, using an initial pressure of 60 psi of hydrogen. After 4 hours
about 80% of the theoretical volume of hydrogen has been absorbed. The
mixture is filtered, an additional 1.5 g of 10% palladium-on-carbon is added
and the mixture is again hydrogenated on the Parr apparatus under the same
conditions. After hydrogenating for an additional 3 hours, the mixture is
filtered and the filtrate is concentrated in vacuo. The residue is stripped twice
with toluene and crystallized with ether-ethanol to give α-(t-butylaminomethyl)-4-hydroxy-3-ureidobenzyl alcohol hydrochloride, MP 214°-
215°C.
brand name
Bronsecur [as the base].
Therapeutic Function
Bronchodilator
carbuterol Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 | trendseenbio@gmail.com | China | 11681 | 58 |
| Shanghai Kewel Chemical Co., Ltd. | 021-64609169 18901607656 | greensnown@163.com | China | 9911 | 50 |
| BOC Sciences | 16314854226 | info@bocsci.com | United States | 9926 | 65 |
| TOSUN PHARM | 020-61855200 13326451905 | 2881290884@qq.com | China | 7487 | 58 |
| Cato Research Chemicals Inc. | 020-81215950 4000-868-328 | customer@uwalab.com | United States | 10413 | 58 |
| ShenZhen Trendseen Biological Technology Co.,Ltd. | 13417589054 13417589054 | trendseenbio@gmail.com | China | 8941 | 58 |
| Shaanxi Xihua Chemical Industry Co., Ltd | 17691182729 15529505138 | 1021@dideu.com | China | 10011 | 58 |
| Hubei Qingbei Yunyan Pharmaceutical Technology Co., Ltd | 18162595016 | 3287908757@qq.com | China | 9887 | 58 |