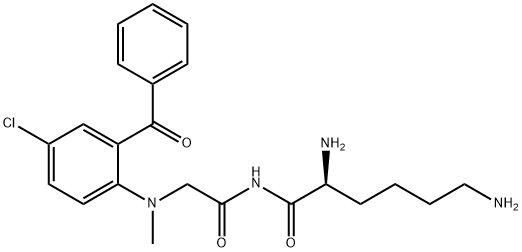
pro-diazepam
- CAS No.
- 65617-86-9
- Chemical Name:
- pro-diazepam
- Synonyms
- pro-diazepam;Ro03-7355/000;Ro-03-7355/000;Ro 03-7355/000;GlycinaMide, L-lysyl-N-(2-benzoyl-4-chlorophenyl)-N-Methyl-
- CBNumber:
- CB61352937
- Molecular Formula:
- C22H27ClN4O3
- Molecular Weight:
- 430.933
- MDL Number:
- MFCD00865312
- MOL File:
- 65617-86-9.mol
| Boiling point | 697.6±55.0 °C(Predicted) |
|---|---|
| Density | 1.253±0.06 g/cm3(Predicted) |
| pka | 13.65±0.46(Predicted) |
| EWG's Food Scores | 1 |
| FDA UNII | 65NK71K78P |
pro-diazepam price
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| TRC | A794675 | Avizafone | 65617-86-9 | 1mg | $165 | 2021-12-16 | Buy |
| TRC | A794675 | Avizafone | 65617-86-9 | 10mg | $1320 | 2021-12-16 | Buy |
| American Custom Chemicals Corporation | API0016551 | AVIZAFONE 95.00% | 65617-86-9 | 5MG | $306.9 | 2021-12-16 | Buy |
pro-diazepam Chemical Properties,Uses,Production
Originator
Avizafone,Onbio Inc.
Uses
Avizafone
Definition
ChEBI: Avizafone is a peptide.
Manufacturing Process
(a) 20.9 g of N-benzyloxycarbonylglycine were suspended in 1500 ml of dry
1,2-dimethoxyethane and the suspension was cooled to -20°C. 10.1 g of Nmethylmorpholine
and 13.7 g of isobutylchloroformate were added, the
resulting solution was stirred at -20°C for 1 hour and then filtered. The filtrate
was added portion-wise over a period of several hours to a refluxing solution
of 24.55 g of 5-chloro-2-methylaminobenzophenone in 200 ml of 1,2-
dimethoxyethane, the resulting mixture was boiled overnight and then
evaporated to dryness in vacuum. The yellow residue was dissolved in ethyl
acetate, washed with two portions of water and one portion of saturated
sodium chloride solution, dried over anhydrous magnesium sulfate and then
evaporated. Column chromatography of the residue on Florisil (Trade Mark)
using mixtures of benzene and chloroform yielded 35 g (80%) of pure 2-(Nbenzyloxycarbonylamino)-
N-(2-benzoyl-4-chlorophenyl)-N-methylacetamide as
a pale yellow gum.
43.7 g of 2-(N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide
were dissolved in 200 ml of a 30% solution of hydrogen
bromide in glacial acetic acid and the resulting solution was stirred overnight
at room temperature. The mixture was added slowly to a large excess (2000
ml) of dry diethyl ether with vigorous stirring. The product, which separated
was allowed to settle and the supernatant liquors were decanted off. The
residue was triturated with 150 ml of acetone and the product filtered off,
washed consecutively with the minimum amount of acetone and dry diethyl
ether and dried in vacuum to give 29.5 g (77%) of 2-amino-N-(2-benzoyl-4-
chlorophenyl)-N'-methylacetamide hydrobromide as a white hygroscopic
powder of melting point 194°-195°C (decomposition).
(b) 84 g of N-benzyloxycarbonylglycine were suspended in 500 ml of alcoholfree
chloroform and the suspension was cooled to -200°C. The stirred
suspension was treated portion-wise over a period of 15 minutes with 90 g of
phosphorus pentachloride and the stirring was continued until a clear solution
was obtained. At this point, the cold mixture was added dropwise over a
period of 30 minutes to a cold (-5°C) vigorously stirred emulsion consisting of
82 g of 5-chloro-2-methylaminobenzophenone, 347 g of potassium
bicarbonate, 700 ml of chloroform and 1400 ml of water. The resulting mixture was stirred for a further 1 hour at -5°C and then overnight at room
temperature. The stirring was then discontinued and the liquid phases allowed
to separate. The chloroform layer was washed three times with 500 ml of
water each time and evaporated in VW to give 150.7 g of a viscous yellow
gum which was shown by physical methods to be almost pure (above 95%) 2-
(N-benzyloxycarbonylamino)-N-(2-benzoyl-4-chlorophenyl)-Nmethylacetamide.
The product above obtained was dissolved in 650 ml of a
30% solution of hydrogen bromide in glacial acetic acid and treated in an
identical manner to that described in part (a) to give 2-amino-N-(2-benzoyl-
4chlorophenyl)-N-methylacetamide hydrobromide in a 77% overall yield from
5-chloro-2-methylaminobenzophenone.
(c) 1 mole of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl N-hydroxysuccinimide ester
was dissolved in 50 ml of dry dimethylformamide. The resulting solution was
cooled to -20°C and there were added 1 mole of 2-amino-N-(2-benzoyl-4-
chlorophenyl)-N-methylacetamide hydrobromide followed by the dropwise
addition of 1 mole of N-ethylmorpholine. The resulting mixture was vigorously
stirred for 1 hour at -20°C and then overnight at room temperature. The
solvent was evaporated in vacuum and the residue dissolved in a mixture of
dichloromethane and water. The organic and aqueous layers were separated
and the aqueous phase extracted with further portions of dichloromethane.
The combined organic phases (250 ml) were washed three times with 50 ml
of water each time, dried over anhydrous magnesium sulfate and evaporated
in vacuum to give a yellow oily residue, which was shown by physical methods
to consist of Nα,Nε-bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)-
N-methylglycinamide as an almost colorless light-sensitive gum, [α]D
20= -9.3°
(c=1 in ethanol).
Nα,Nε-Bisbenzyloxycarbonyl-L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide
was converted using a 30% solution of hydrogen bromide
in glacial acetic acid into L-lysyl-N-(2-benzoyl-4-chlorophenyl)-Nmethylglycinamide
dihydrobromide which was obtained as a hygroscopic
powder of melting point 145°C (decomposition), [α]D
20= + 15.6° (c=1 in
water).
Treatment of the foregoing dihydrobromide in aqueous solution by passage
over an excess of an anion-exchange resin such as 'Amberlite' IRA-401 in the
chloride form followed by lyophilisation of the eluate gave, in quantitative
yield, L-lysyl-N-(2-benzoyl-4-chlorophenyl)-N-methylglycinamide
dihydrochloride (avizafone) as a hygroscopic white light-sensitive powder of
melting point 125°-145°C (slow decomposition), [α]D
20= +19.3° (c= 1 in
water).
Therapeutic Function
Anxiolytic, Anticonvulsant
pro-diazepam Preparation Products And Raw materials
Raw materials
1of2
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| ANHUI SHENGZHIKAI BIOTECHNOLOGY CO.,LTD | +8619155187336 | shengzhikai3@shengzhikai.com | China | 38 | 58 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Zhejiang J&C Biological Technology Co.,Limited | +1-2135480471 +1-2135480471 | sales@sarms4muscle.com | China | 10523 | 58 |
| Hebei Miaoyin Technology Co.,Ltd | +86-17367732028 +86-17367732028 | kathy@hbyinsheng.com | China | 3582 | 58 |
| Jiangsu shring Biopharma Co., Ltd. | +8613372282299 | sales@shringchem.com | China | 3545 | 58 |
| J & K SCIENTIFIC LTD. | 010-82848833 400-666-7788 | jkinfo@jkchemical.com | China | 96815 | 76 |
| Hangzhou Sage Chemical Co., Ltd. | +86057186818502 13588463833 | info@sagechem.com | China | 10269 | 58 |
| Shanghai TaoSu Biochemical Technology Co., Ltd. | 021-33632979 | info@tsbiochem.com | China | 8073 | 58 |
| AN PharmaTech Co Ltd | 86(21)68097365 | sales@anpharma.net | China | 4901 | 55 |