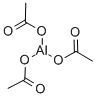
Aluminum acetate
- CAS No.
- 139-12-8
- Chemical Name:
- Aluminum acetate
- Synonyms
- D02843;Domeboro;Aluminumaceate;ALUMNIM ACETATE;aluminiumacetate;BUROW'S SOLUTION;ALUMINUM ACETATE;ALUMININUM ACETATE;aluminium(tri)acetate;Aluminum acetate (usp)
- CBNumber:
- CB6418335
- Molecular Formula:
- C6H9AlO6
- Molecular Weight:
- 204.11
- MDL Number:
- MFCD00078833
- MOL File:
- 139-12-8.mol
| Melting point | decomposes with moisture [CRC10] |
|---|---|
| Density | 1.002 g/cm3 |
| solubility | soluble in H2O; slightly soluble in acetone |
| color | white hygroscopic solid |
| Water Solubility | soluble H2O [HAW93] |
| LogP | -0.285 (est) |
| Indirect Additives used in Food Contact Substances | ALUMINUM ACETATE |
| FDA 21 CFR | 175.105; 176.170 |
| EWG's Food Scores | 3-4 |
| FDA UNII | 80EHD8I43D |
| EPA Substance Registry System | Acetic acid, aluminum salt (139-12-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H315 |
| Precautionary statements | P264-P280-P302+P352-P321-P332+P313-P362-P264-P280-P305+P351+P338-P337+P313P |
Aluminum acetate Chemical Properties,Uses,Production
Chemical Properties
Aluminum acetate, AL(C2H302)3,is white crystals, soluble, by reaction of aluminum hydroxide and acetic acid and then crystallizing. Used as a mordant in dyeing and printing textiles, in the manufacturing of lakes, for fire proofing fabrics, for waterproofing cloth.
Description
Ammonium acetate is a chemical compound with the formula NH4C2H3O2 (or C2H4O2.NH3 or C2H7NO2). It is a white solid and can be derived from the reaction of ammonia and acetic acid. It is available commercially and, depending on grade, can be rather inexpensive.
Description
Aluminum acetate is a chemical compound and is a salt which can be produced by the reaction of aluminum hydroxide and acetic acid. The compound formula for Aluminum Acetate is Al(CH3COO)3.
Chemical Properties
white powder(s); preparation: by heating aluminum or AlCl3 with an acetic acid solution containing acetic anhydride; uses: as an antiseptic, an astringent, and in antiperspirant applications; there is a hydroxyaluminum diacetate, CAS RN 142-03-0 [CIC73] [HAW93] [ALD94]
Chemical Properties
CH3COONH4 is hygroscopic and decomposes easily to acetamide if heated above 165 °C.
CH3COONH4 → CH3C(O)NH2 + H2O
In this reaction, a salt is converted to two molecular species, which is a relatively uncommon conversion at mild temperatures.
Uses
Astringent.
Production Methods
Ammonium acetate is produced by the neutralization of acetic acid with ammonium carbonate or by saturating glacial acetic acid with dry ammonia gas. Obtaining crystalline ammonium acetate is difficult on account of its aqueous solution giving off ammonia when evaporated.
Definition
(aluminum acetate; Al(OOCCH3)3) A white solid soluble in water. It is usually obtained as the dibasic salt, basic aluminum ethanoate, Al(OH)(CH3COO)2. It is prepared by dissolving aluminum hydroxide in ethanoic acid and is used extensively as a mordant in dyeing and as a size for paper and cardboard products. The solution is hydrolyzed and contains various complex aluminumhydroxyl species and colloidal aluminum hydroxide.
Application
The diacetate is used as an antiseptic. The Aluminum Acetate compound can be used medicinally to treat infections in the outer ear canal. It is used in the name brand drug Domeboro, which contains acetic acid/aluminum acetate.This medication kills the infectious bacteria and fungus as well as drying out the ear canal.
Since it acts as a drying agent, it can also be used in the treatment of severe rashes, such as poison ivy, poison oak, and poison sumac.
Definition
A salt obtained by reaction of aluminum hydroxide and acetic acid with subsequent recrystallization. Its neutral form Al(C 2 H 3 O 2 ) 3 is a white, water-soluble powder used in solution as an antiseptic, astringent, and antiperspirant. Its basic form is Al(C 2 H 3 O 2 ) 2 OH, also known as aluminum diacetate and aluminum subacetate. It is a crystalline solid, insoluble in water, used as a mordant in textile dyeing, as a flame retardant and waterproofing agent, and in manufacture of lakes and pigments.
brand name
Buro-Sol Concentrate (Doak); Domeboro (Bayer).
Industrial uses
As the salt of a weak acid and a weak base, ammonium acetate has a number of distinctive properties.
NH4C2H3O2 is occasionally employed as a biodegradable deicing agent.
It is often used with acetic acid to create a buffer solution, one that can be thermally decomposed to non-ionic products
Ammonium acetate is useful as a catalyst in the Knoevenagel condensation and as a source of ammonia in the Borch reaction in organic synthesis.
It is a relatively unusual example of a salt that melts at low temperatures.
Can be used with distilled water to make a protein precipitating reagent.
Is often used as an aqueous buffer for ESI mass spectrometry of proteins and other molecules.
Ammonium acetate is volatile at low pressures. Because of this it has been used to replace cell buffers with non-volatile salts, in preparing samples for mass spectrometry.It is also popular as a buffer for mobile phases for HPLC with ELSD detection for this reason. Other volatile salts that have been used for this include ammonium formate.
Synthesis
The triacetate forms when aluminum sulfate is mixed with barium acetate. Another synthetic method is by bringing together aluminum hydroxide, acetic anhydride and glacial acetic acid in water, forming the basic aluminum monoacetate
The diacetate is prepared in a reaction of sodium aluminate (NaAlO2) with acetic acid.
Aluminum acetate Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hangzhou Bayee Chemical Co., Ltd. | 0086-571-86990109 | rachelhoo@bayeechem.com | China | 104 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21691 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 | sales@conier.com | China | 49390 | 58 |
| career henan chemical co | +86-0371-86658258 15093356674; | factory@coreychem.com | China | 29826 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-81148696 +8615536356810 | 1047@dideu.com | China | 3408 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Zhengzhou Alfa Chemical Co.,Ltd | +8618530059196 | sale04@alfachem.cn | China | 12468 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
View Lastest Price from Aluminum acetate manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2023-05-30 | Aluminum acetate
139-12-8
|
US $0.00-0.00 / kg | 1kg | 99% | 500000kg | Hebei Kingfiner Technology Development Co. , Ltd. | |
 |
2020-03-06 | Aluminum acetate
139-12-8
|
US $1.00 / KG | 1KG | 99% | 200kg | Career Henan Chemical Co | |
 |
2020-01-05 | Aluminum acetate
139-12-8
|
US $0.10 / KG | 1KG | 99.0% | 1000 tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Aluminum acetate
139-12-8
- US $0.00-0.00 / kg
- 99%
- Hebei Kingfiner Technology Development Co. , Ltd.
-

- Aluminum acetate
139-12-8
- US $1.00 / KG
- 99%
- Career Henan Chemical Co
-

- Aluminum acetate
139-12-8
- US $0.10 / KG
- 99.0%
- Shaanxi Dideu Medichem Co. Ltd