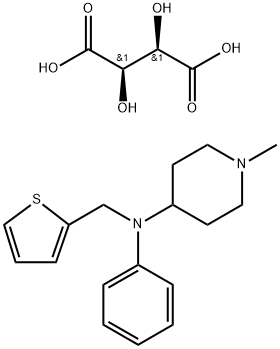
thenalidine tartrate
- CAS No.
- 2784-55-6
- Chemical Name:
- thenalidine tartrate
- Synonyms
- Sandosten Tartrate;thenalidine tartrate;(1-methyl-4-piperidyl)-phenyl-(2-thienylmethyl)amine;1-methyl-N-phenyl-N-(thiophen-2-ylmethyl)piperidin-4-amine
- CBNumber:
- CB6919423
- Molecular Formula:
- C21H28N2O6S
- Molecular Weight:
- 436.52
- MDL Number:
- MFCD28977561
- MOL File:
- 2784-55-6.mol
| FDA UNII | H581Q95SJA |
|---|
thenalidine tartrate Chemical Properties,Uses,Production
Originator
Thenalidine tartarte,Sandoz (Novartis)
Manufacturing Process
12 parts by weight of 1-methyl-4-amino-N'-phenyl-piperidine are dissolved in
the five- to six-fold quantity of absolute xylene and then, while refluxing and
stirring the resultant solution, 42.92 parts by weight of sodamide (10%
excess) are added in the course of 2 to 3 hours. Then, without interrupting
the heating, 144.5 parts by weight of freshly distilled 2-thenyl chloride,
dissolved in the two-fold quantity of absolute xylene, are added dropwise in
the course of 1.5 hours, the mixture being there upon heated for 40 to 42
hours at an oil-bath temperature of 170°C. After the mixture has cooled, any
sodium amide which is present is decomposed with 10 to 20 parts by weight
of NH4CI, xylene is added, and the mixture shaken out with about 600 parts
by volume of water. The aqueous extract is clarified by filtration and then
shaken out with benzene. The xylene and; benzene extracts are concentrated
by evaporation under reduced pressure. Any remaining unreacted 2-thenyl
chloride removed at 110°C/11 mm. The residue from the evaporation then
distilled at a pressure of 0.1 mm. Unreacted 1-methyl-4-amino-N'-
phenylpiperidine distils over first at 110-120°C, followed by impure 1-methyl-4-amino-N'-phenyl-N'-(2-thenyl)-piperidine at 180-190°C.
In order to purify the latter compound, the crude base is dissolved in the sixfold
quantity of absolute alcohol. A five-fold quantity of an absolute alcoholic
solution of oxalic acid containing the stoichiometric quantity of oxalic acid (+
10% excess) to form the monooxalate, is their added. A considerable
evolution of heat taking place. Upon cooling of the reaction mixture; the
monooxalate crystallizes out slowly, in an 80% yield. For purification
purposes, the thus-obtained monooxalate is recrystallized from the 17-fold
quantity (by volume) of absolute alcohol, with addition of animal charcoal,
followed by recrystaillization from the 16-fold quantity (by volume) of a
mixture of alcohol and benzene (1:1). The purified monooxalate melts at 160-
162°C (decomposition).
To obtain the pure base, the oxalate is dissolved at 40°C in the twenty-fold
quantity (by volume) of water and, while cooling with ice-water, the solution is
rendered alkaline with 3-normal aqueous NaOH solution. The base, which at
first separates in the form of a milky precipitate, crystallizes in the course of
several hours and is then recrystallized from the 12-fold quantity (by volume)
of an alcohol-water mixture (7.5:4.5). The purified base melts at 95-97°C.
In practice it is usually used as tartrate salt.
Therapeutic Function
Antihistaminic, Antipruritic
thenalidine tartrate Preparation Products And Raw materials
thenalidine tartrate Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Lanospharma Laboratories Co.,Ltd | -- | sales@lanospharma.com | China | 6343 | 56 |
| CHEMICAL LAND21 | -- | sales21@chemicalland21.com | South Korea | 6315 | 74 |
| Supplier | Advantage |
|---|---|
| Lanospharma Laboratories Co.,Ltd | 56 |
| CHEMICAL LAND21 | 74 |