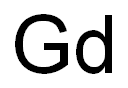GADOLINIUM
- CAS No.
- 7440-54-2
- Chemical Name:
- GADOLINIUM
- Synonyms
- Gd;GD007910;GD007925;GD005105;GD007905;GADOLINIUM;Gadolinium foil;gadolinium atom;GADOLINIUM METAL;Gadolinium chips
- CBNumber:
- CB7393251
- Molecular Formula:
- Gd
- Molecular Weight:
- 157.25
- MDL Number:
- MFCD00011022
- MOL File:
- 7440-54-2.mol
- MSDS File:
- SDS
| Melting point | 1313 °C (lit.) |
|---|---|
| Boiling point | 3273 °C (lit.) |
| Density | 7.886 g/mL at 25 °C (lit.) |
| Flash point | 3266°C |
| solubility | soluble in dilute acid solutions |
| form | ingot |
| color | Silver |
| Specific Gravity | 7.898 |
| Resistivity | 126 μΩ-cm, 20°C |
| Water Solubility | Soluble in magnesium and diluted acid. Insoluble in water. |
| Sensitive | Air & Moisture Sensitive |
| Merck | 13,4347 |
| Exposure limits |
ACGIH: TWA 2 ppm; STEL 4 ppm OSHA: TWA 2 ppm(5 mg/m3) NIOSH: IDLH 25 ppm; TWA 2 ppm(5 mg/m3); STEL 4 ppm(10 mg/m3) |
| Stability | Stable. Powder is highly flammable. Incompatible with strong oxiziding agents, halogens, acids. Powder may react with water or moisture. |
| CAS DataBase Reference | 7440-54-2(CAS DataBase Reference) |
| EWG's Food Scores | 1 |
| NCI Dictionary of Cancer Terms | gadolinium |
| FDA UNII | AU0V1LM3JT |
| EPA Substance Registry System | Gadolinium (7440-54-2) |
| Poissons Ratio | 0.259 |
|---|---|
| Shear Modulus | 21.8 GPa, Calculated |
| Hardness, Vickers | 37, polycrystalline |
| Bulk Modulus | 37.9 GPa |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS02 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H228-H261 | |||||||||
| Precautionary statements | P210-P231+P232-P240-P241-P280-P370+P378 | |||||||||
| Hazard Codes | F,Xi | |||||||||
| Risk Statements | 15-36/38 | |||||||||
| Safety Statements | 43-7/8-26-Neverusewater. | |||||||||
| RIDADR | UN 3208 4.3/PG 3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | LW3850000 | |||||||||
| F | 1 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | III | |||||||||
| HS Code | 2846902015 | |||||||||
| NFPA 704 |
|
GADOLINIUM price More Price(78)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 263060 | Gadolinium 40mesh, 99% trace rare earth metals basis | 7440-54-2 | 5g | $169 | 2024-03-01 | Buy |
| Sigma-Aldrich | 261114 | Gadolinium ingot, 99.9% trace rare earth metals basis | 7440-54-2 | 10g | $137 | 2023-01-07 | Buy |
| Alfa Aesar | 088065 | Gadolinium, AAS standard solution | 100mL | $34.65 | 2024-03-01 | Buy | |
| Alfa Aesar | 088065 | Gadolinium, AAS standard solution | 500mL | $87.65 | 2024-03-01 | Buy | |
| Alfa Aesar | 014373 | Gadolinium, plasma standard solution | 100mL | $117.65 | 2024-03-01 | Buy |
GADOLINIUM Chemical Properties,Uses,Production
Description
Gadolinum is found in minerals bastnasite and monazite, always associated with other rare earth metals. It was isolated from yttria in 1880 by the Swiss chemist Marignac, and discovered independently in 1885 by Boisbaudran. It was named in honor of the Swedish chemist Gadolin. Its abundance in the earth’s crust is 6.2 mg/kg and concentration in sea water is 0.7 ng/L.
Gadolinium is also found in alloys and special minerals known as yttrium garnets. An alloy is made by melting and mixing two or more metals. The mixture has properties different from those of the individual metals. Gadolinium alloys are easier to work with than alloys without gadolinium. Gadolinium yttrium garnets are used in microwave ovens to produce the microwaves. Gadolinium metal is not especially reactive. It dissolves in acids and reacts slowly with cold water. It also reacts with oxygen at high temperatures.
The most important application of this metal is as control rod material for shielding in nuclear power reactors. Its thermal neutron absorption cross section is 46,000 barns. Other uses are in thermoelectric generating devices, as a thermoionic emitter, in yttrium-iron garnets in microwave filters to detect low intensity signals, as an activator in many phosphors, for deoxidation of molten titanium, and as a catalyst. Catalytic applications include decarboxylation of oxaloacetic acid; conversion of ortho- to para-hydrogen; and polymerization of ethylene.
Uses
Gadolinium oxide (Gd2O3) has multiple uses in medicine, chemical processes, electronics, and glass making. Gadolinium oxide is used in the creation of the phosphors used in television tubes as well as the creation of gadolinium yttrium garnets used in microwaves and materials used to absorb atomic reactions. Gadolinium has a shiny metallic lustre with a slight yellowish tint. It is both ductile and malleable.
Gadolinium is utilized for both its high magnetic moment (7.94uB) and in phosphors and scintillated material. When mixed with EDTA dopants, it is used as an injectable contrast agent for patients undergoing magnetic resonance imaging. With its high magnetic moment, Gadolinium can reduce relaxation times and thereby enhance signal intensity.
Gadolinium also possesses unusual metallurgic properties, with as little as 1% of Gadolinium improving the workability and resistance of Iron, Chromium, and related alloys to high temperatures and oxidation.
Gadolinium compounds are also used for making green phosphors for color TV tubes.
Gadolinium is used for making Gadolinium Yttrium Garnet (Gd:Y3Al5O12); it has microwave applications and is used in fabrication of various optical components and as substrate material for magneto-optical films.
Gadolinium Metal is ferromagnetic, ductile and malleable metal, and widely used for making speciality alloys, MRI(magnetic Resonance Imaging), superconductive materials and magnetic refrigerator. Gadolinium is also used in nuclear marine propulsion systems as a burnable poison. In X-ray systems, gadolinium is contained in the phosphor layer, suspended in a polymer matrix at the detector. It is used for making Gadolinium Yttrium Garnet (Gd:Y3Al5O12); it has microwave applications and is used in fabrication of various optical components and as substrate material for magneto-optical films. Gadolinium Gallium Garnet (GGG, Gd3Ga5O12) was used for imitation diamonds and for computer bubble memory. It can also serve as an electrolyte in Solid Oxide Fuel Cells (SOFCs).
Reactions
The only oxidation state known for this metal is +3. Therefore, all its compounds are trivalent. It reacts with dilute mineral acids forming the corresponding salts. The reaction is vigorous but usually not violent.
2Gd + 3H2SO4 → Gd2(SO4)3 + 3H2
2Gd + 6HCl → 2GdCl3 + 3H2
Although the metal is stable in air at ordinary temperature, it burns in air when heated at 150 to 180°C, particularly when present in sponge or powdered form having a large surface area. The product is gadolinium(III) oxide, Gd2O3.
Gadolinium is a strong reducing agent. It reduces oxides of several metals such as iron, chromium, lead, manganese, tin, and zirconium into their elements. The standard oxidation potential for the reaction
Gd → Gd3+ + 3e– is 2.2 volts.
Gadolinium burns in halogen vapors above 200°C forming gadolinium(III) halides:
2Gd + 3Cl2 → 2GdCl3
When heated with sulfur, the product is gadolinium sulfide Gd2S3. Similarly, at elevated temperatures, gadolinium combines with other nonmetals such as nitrogen, hydrogen, and carbon forming nitride, hydride, and carbide respectively:
2Gd + N2 → 2GdN
2Gd + 3H2 → 2GdH3
Production Methods
Gadolinium is produced from both its ores, monazite and bastnasite. After the initial steps of crushing and beneficiation, rare earths in the form of oxides are attacked by sulfuric or hydrochloric acid. Insoluble rare earth oxides are converted into soluble sulfates or chlorides. When produced from monazite sand, the mixture of sand and sulfuric acid is initially heated at 150°C in cast iron vessels. Exothermic reaction sustains the temperature at about 200 to 250°C. The reaction mixture is cooled and treated with cold water to dissolve rare earth sulfates. The solution is then treated with sodium pyrophosphate to precipitate thorium. Cerium is removed next. Treatment with caustic soda solution followed by air drying converts the metal to cerium(IV) hydroxide. Treatment with hydrochloric or nitric acid solGADOLINIUM 303ubilizes all rare earths except cerium. Rare earth salt solution is then treated with magnesium nitrate. The double salts of samarium, europium, and gadolinium nitrate crystallize out. Individual salts are separated by ion exchange methods.
Gadolinium is obtained from its salts, usually its chloride or fluoride, by heating with excess calcium at 1,450°C under argon. The reduction is carried out in a tantalum crucible. Alternatively, fused gadolinium chloride mixed with sodium or potassium chloride is electrolyzed in an iron pot that serves as the anode and using a graphite cathode. Sponge gadolinium may be produced by reducing molten gadolinium chloride with a reducing metal oxide in vaporized state at a temperature below 1,300°C (the melting point of gadolium) at a reduced pressure.
Chemical Properties
metal foil, chunks or powder. The powder of gadolinium is highly flammable; incompatible with strong oxidising agents, halogens, acids; and reacts with water or moisture.
Physical properties
Gadolinium is silvery-white, soft, malleable, and ductile with a metallic luster. It is the secondof what is referred to as the dysprosium, subgroup in the middle of the lanthanide seriesof rare-earths. It tarnishes in air, forming the oxide (Gd2O3) on the surface, which flakes offthe surface, exposing a fresh metal that in turn oxidizes.
Its melting point is 1,313°C, its boiling point is 3,273°C, and its density is 7.90g/cm3.
Isotopes
There are 39 isotopes of gadolinium. Seven of these are stable. They are: Gd-54, which makes up 2.18% of all the gadolinium found in the Earth’s crust; Gd-55,supplying 14.80%; Gd-156, making up 20.47%; Gd-157, constituting 15.56%; and Gd-158, accounting for 24.85%. In addition, there are two isotopes of gadolinium that areradioactive and with such long half-lives that they still exist in the Earth’s crust. They areregarded as stable isotopes along with the other seven. They are Gd-152 (1.08×10+14years), which exists in just 0.20% in abundance, and Gd-160 (1.3×10+21 years), foundin 21.86% abundance.
Origin of Name
Named for the mineral gadolinite, which was named for the French chemist Johann Gadolin.
Occurrence
Gadolinium is the 40th most abundant element on Earth and the sixth most abundant ofthe rare-earths found in the Earth’s crust (6.4 ppm). Like many other rare-earths, gadoliniumis found in monazite river sand in India and Brazil and the beach sand of Florida as well asin bastnasite ores in southern California. Similar to other rare-earths, gadolinium is recoveredfrom its minerals by the ion-exchange process. It is also produced by nuclear fission in atomicreactors designed to produce electricity.
Characteristics
Gadolinium, unlike most of the rare earths in the dysprosium subgroup, reacts slowlywith water, releasing hydrogen. It is strongly magnetic at low temperatures. Two of its stableisotopes (Gd-155 and Gd-157) have the greatest ability of all natural elements to absorb thermalneutrons to control the fission chain reaction in nuclear reactors. However, few of theseisotopes are found in the ores of gadolinium.
History
Gadolinia, the oxide of gadolinium, was separated by Marignac in 1880 and Lecoq de Boisbaudran independently isolated Gadolinium from Mosander’s “yttria” in 1886. The element was named for the mineral gadolinite from which this rare earth was originally obtained. Gadolinium is found in several other minerals, including monazite and bastnasite, which are of commercial importance. With the development of ion-exchange and solvent extraction techniques, the availability and price of gadolinium and the other rare-earth metals have greatly improved. Thirtyone isotopes and isomers of gadolinium are now recognized; seven are stable and occur naturally. The metal can be prepared by the reduction of the anhydrous fluoride with metallic calcium. As with other related rare-earth metals, it is silvery white, has a metallic luster, and is malleable and ductile. At room temperature, gadolinium crystallizes in the hexagonal, close-packed α form. Upon heating to 1235°C, α gadolinium transforms into the β form, which has a body-centered cubic structure. The metal is relatively stable in dry air, but in moist air it tarnishes with the formation of a loosely adhering oxide film which splits off and exposes more surface to oxidation. The metal reacts slowly with water and is soluble in dilute acid. Gadolinium has the highest thermal neutron capture cross-section of any known element (49,000 barns). Natural gadolinium is a mixture of seven isotopes. Two of these, 155Gd and 157Gd, have excellent capture characteristics, but they are present naturally in low concentrations. As a result, gadolinium has a very fast burnout rate and has limited use as a nuclear control rod material. It has been used in making gadolinium yttrium garnets, which have microwave applications. Compounds of gadolinium are used in making phosphors for color TV tubes. The metal has unusual superconductive properties. As little as 1% gadolinium has been found to improve the workability and resistance of iron, chromium, and related alloys to high temperatures and oxidation. Gadolinium ethyl sulfate has extremely low noise characteristics and may find use in duplicating the performance of amplifiers, such as the maser. The metal is ferromagnetic. Gadolinium is unique for its high magnetic moment and for its special Curie temperature (above which ferromagnetism vanishes) lying just at room temperature. This suggests uses as a magnetic component that senses hot and cold. The price of the metal is about $5/g (99.9% purity).
Uses
Oxide of Gadolinium foil is used in the control rods of some nuclear reactors. It is used as nuclear control rod material. Neutron shielding, phosphor activator, catalyst, scavenger for oxygen in titanium production. A metal element that is used in magnetic resonance imaging (MRI) and other imaging methods. It has been used in making gadolinium yttrium garnets, which have microwave applications.
Uses
Gadolinium’s main use is based on its ability to absorb neutrons, thus making it ideal as aneutron-shielding and neutron-absorbing metal. It is also used as an alloying agent for steel andother metals to make the metals more workable and to be able to withstand low temperatures.
Gadolinium is used in the manufacture of electronics and can be combined with yttriumto make garnets used in microwaves. Gadolinium is used as a catalyst to speed up chemicalreactions, and to activate phosphor compounds in TV screens and cast filaments in electricaldevices. It is also used in high-temperature furnaces. Gadolinium is paramagnetic at normalroom temperatures (weaker than ferromagnetic) and becomes strongly ferromagnetic at verycold temperatures.
Uses
Neutron shielding, garnets in microwave filters, phosphor activator, catalyst, scavenger for oxygen in titanium production
Definition
A rare-earth element of the lanthanide series, atomic number 64, group IIIB of the periodic table, aw 157.25, valence of 3; seven natural isotopes.
Definition
gadolinium: Symbol Gd. A soft silverymetallic element belonging tothe lanthanoids; a.n. 64; r.a.m.157.25; r.d. 7.901 (20°C); m.p. 1313°C;b.p. 3266°C. It occurs in gadolinite,xenotime, monazite, and residuesfrom uranium ores. There are sevenstable natural isotopes and elevenartificial isotopes are known. Two ofthe natural isotopes, gadolinium–155and gadolinium–157, are the bestneutron absorbers of all the elements.The metal has found limitedapplications in nuclear technologyand in ferromagnetic alloys (withcobalt, copper, iron, and cerium).Gadolinium compounds are used inelectronic components. The elementwas discovered by Jean de Marignac(1817–94) in 1880.
Definition
A ductile malleable silvery element of the lanthanoid series of metals. It occurs in association with other lanthanoids. Gadolinium is used in alloys, magnets, and in the electronics industry. Symbol: Gd; m.p. 1313°C; b.p. 3266°C; r.d. 7.9 (25°C); p.n. 64; r.a.m. 157.25.
Hazard
The halogens of gadolinium are very toxic, and gadolinium nitrate is explosive. As withmost rare-earths, care should be taken not to inhale fumes or ingest particles of gadolinium.
Flammability and Explosibility
Flammable
GADOLINIUM Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| career henan chemical co | +86-0371-86658258 | sales@coreychem.com | China | 29914 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 | sales@chemdad.com | China | 39916 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 | sales@tnjchem.com | China | 34572 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-029-89586680 +86-18192503167 | 1026@dideu.com | China | 9271 | 58 |
| Zhuoer Chemical Co., Ltd | 02120970332; +8613524231522 | sales@zhuoerchem.com | China | 3015 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 | alfa4@alfachem.cn | China | 12755 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 | 2571773637@qq.com | China | 9821 | 58 |
| Alfa Chemistry | +1-5166625404 | Info@alfa-chemistry.com | United States | 21317 | 58 |
| LEAP CHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 24738 | 58 |
View Lastest Price from GADOLINIUM manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2019-07-06 | GADOLINIUM
7440-54-2
|
US $1.00 / kg | 1kg | 99% | Customized | Career Henan Chemical Co |
-

- GADOLINIUM
7440-54-2
- US $1.00 / kg
- 99%
- Career Henan Chemical Co
7440-54-2(GADOLINIUM)Related Search:
1of4