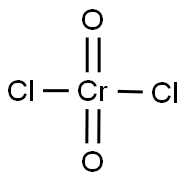
Chromyl chloride
- CAS No.
- 14977-61-8
- Chemical Name:
- Chromyl chloride
- Synonyms
- chromyl;Cl2CrO2;Chromylchlorid;Chromoxychlorid;CHROMYL CHLORIDE;(beta-4)-chromiu;Chroomoxychloride;Chromyldichloride;cromile,clorurodi;chromylchlorid[qr]
- CBNumber:
- CB7853128
- Molecular Formula:
- Cl2CrO2
- Molecular Weight:
- 154.9
- MDL Number:
- MFCD00010951
- MOL File:
- 14977-61-8.mol
| Melting point | -96.5 °C (lit.) |
|---|---|
| Boiling point | 117 °C (lit.) |
| Density | 1.911 g/mL at 25 °C (lit.) |
| Flash point | 117°C |
| solubility | reacts with H2O; soluble in ctc, chloroform,benzene |
| form | liquid |
| Specific Gravity | 1.911 |
| color | red |
| Water Solubility | Soluble in carbon tetrachloride, carbon disulfide, benzene, nitrobenzene, chloroform, and POCL3. Insoluble in water(Reacts). |
| Sensitive | Moisture Sensitive |
| Merck | 14,2248 |
| Dielectric constant | 2.6(20℃) |
| Exposure limits |
ACGIH: TWA 0.0001 ppm; STEL 0.00025 ppm (Skin) NIOSH: TWA 0.0002 mg/m3 |
| CAS DataBase Reference | 14977-61-8(CAS DataBase Reference) |
| FDA UNII | JQU316FZ5W |
| EPA Substance Registry System | Chromium(VI) dioxychloride (14977-61-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |      GHS03,GHS05,GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H271-H314-H317-H340-H350-H410 | |||||||||
| Precautionary statements | P210-P273-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338 | |||||||||
| Hazard Codes | O,T,C,N | |||||||||
| Risk Statements | 49-46-8-35-43-50/53-34-23/24/25-45 | |||||||||
| Safety Statements | 53-45-60-61-36/37/39-27-26-17 | |||||||||
| RIDADR | UN 3244 8/PG 2 | |||||||||
| OEB | E | |||||||||
| OEL | TWA: 0.001 mg Cr(VI)/m3 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | GB5775000 | |||||||||
| F | 8-10-21 | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 8 | |||||||||
| PackingGroup | I | |||||||||
| HS Code | 2827499090 | |||||||||
| NFPA 704 |
|
Chromyl chloride price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | 200042 | Chromyl chloride ≥99.99% trace metals basis | 14977-61-8 | 10g | $101 | 2024-03-01 | Buy |
| Alfa Aesar | 040517 | Chromyl chloride, 99.99% (metals basis) | 14977-61-8 | 10g | $103.65 | 2024-03-01 | Buy |
| Alfa Aesar | 040517 | Chromyl chloride, 99.99% (metals basis) | 14977-61-8 | 50g | $464.65 | 2024-03-01 | Buy |
| Strem Chemicals | 93-2409 | Chromyl chloride, 99.5% | 14977-61-8 | 5g | $56 | 2024-03-01 | Buy |
| Strem Chemicals | 93-2409 | Chromyl chloride, 99.5% | 14977-61-8 | 25g | $223 | 2024-03-01 | Buy |
Chromyl chloride Chemical Properties,Uses,Production
Description
Chromyl chloride is a dark red fuming liquidwith a musty, burning odor. Molecular weight = 154.90;Specific gravity (H2O:1) =1.91 at 25℃; Boilingpoint =117℃; Freezing/Melting point =-96.5℃; Vaporpressure =20 mmHg at 25℃. Soluble in water(decomposes).
Chemical Properties
DEEP RED LIQUID
Chemical Properties
Chromyl chloride is a dark red fuming liquid with a musty, burning odor.
Physical properties
Dark red, fuming liquid; reddish yellow vapors; musty buring odor; density 1.91 g/mL; freezes at -96.5°C; boils at 117°C; reacts with water; soluble in chloroform, carbon tetrachloride, benzene, carbon disulfide and nitrobenzene.
Uses
In organic oxidations and chlorinations; as a solvent for chromium oxide; in making chromium complexes and dyes
Uses
Highly spin-polarized chromium dioxide (CrO2) thin films were deposited on 100 TiO2 substrates by chemical vapor deposition using chromyl chloride as a precursor. Chromyl chloride (Cr02C12) reacts with cyclohexane solvent at 75 OC to give a dark precipitate along with chlorocyclohexane and a small amount of cyclohexene (in 10.0 and ca. 0.3% yields based on chromium). Chromyl chloride, CrO2C12, oxidizes cyclooctane, isobutane, and toluene under mild conditions (25-60" C). The reactions give chlorinated products (chlorocyclooctane, tert- butyl chloride, and benzyl chloride) and a dark chromium-containing precipitate. Used for Organic oxidations and chlorination, chromium coordination complexes, catalyst for polymerization of olefins. Chromyl chloride in carbon tetrachloride solution reacts in the cold with cyclohexene, cyclopentene, and 1-hexene to give the various isomeric chlorohydrins as the major products.
Uses
Catalyst for polymerization of olefins; oxidation of hydrocarbons; in the Etard reaction for production of aldehydes and ketones; in the preparation of various coordination complexes of Cr.
Preparation
Chromyl chloride is prepared by reacting chromium(III) chloride with hydrochloric acid:
CrO3 + 2HCl → CrO2Cl2 + H2O
Also, it may be prepared by warming potassium dichromate with potassium chloride in concentrated sulfuric acid:
K2Cr2O7 + 4KCl + 3H2SO4 → 2Cr2O2Cl2 + 3K2SO4 + 3H2O.
Definition
A dark red covalent liquid prepared either by distilling a dry mixture of potassium dichromate and sodium chloride with concentrated sulfuric acid or by the action of concentrated sulfuric acid on chromium(VI) oxide dissolved in concentrated hydrochloric acid. Chromyl chloride is hydrolyzed by water, and with solutions of alkalis it undergoes immediate hydrolysis to produce chromate ions. It is used as an oxidizing agent in organic chemistry. Chromyl chloride oxidizes methyl groups at the ends of aromatic side chains to aldehyde groupings (étard’s reaction).
General Description
A dark red fuming liquid with a pungent odor. Corrosive to tissue.
Reactivity Profile
Chromyl chloride is a powerful and often violent oxidizing agent. Reacts readily with many inorganic and organic materials in the absence of a dilutent. Contact with hydrogen sulfide or phosphine can cause ignition. Contact with phosphorus tribromide, acetone, ethanol, ether, and turpentine causes ignition. Contact with moist phosphorus or with phosphorus trichloride leads to explosive reaction. Contact with ammonia causes incandescence. Reacts with sodium azide to form chromyl azide, which is explosive in the absence of a dilutent. Causes ignition of flowers of sulfur and of urea on contact. [Bretherick, 1979, p. 822-823].
Hazard
Corrosive to tissue. Strong oxidizing agent. Skin and upper respiratory tract irritant. Probable carcinogen.
Health Hazard
Inhalation causes severe irritation of upper respiratory system. Contact with eyes or skin causes irritation and burning. Ingestion causes burning of mouth and stomach.
Fire Hazard
Behavior in Fire: Vapors are very irritating to eyes and mucous membranes. May increase severity of fire.
Safety Profile
Suspected carcinogen. Probably a poison by various routes. Mutation data reported. Corrosive. A strong irritant. Hydrolyzes to form chromic and hydrochloric acids. A strong oxidner and chlorinating agent. Violent reaction with water. Reacts violently with alcohol, ether, acetone, turpentine. Ignites or explodes on contact with nonmetal halides (e.g., disulfur dichloride, phosphorus trichloride, and phosphorus tribromide), nonmetal hydrides (e.g., hydrogen sulfide and hydrogen phosphide), flowers of sulfur, moist phosphorus, sodium azide, and urea. During preparation can violently explode. Incompatible with ammonia, disulfur dichloride, organic solvents, phosphorus, phosphorus trichloride, sodium azide, and sulfur. When heated to decomposition it emits toxic fumes of Cl-. See also CHROMIUM COMPOUNDS.
Potential Exposure
Chromium oxychloride is used in making chromium complexes and dyes; and used in various organic oxidation and chlorination reactions
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. If victim is conscious, administer water ormilk. Do not induce vomiting.
storage
Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.Prior to working with Chromium chloride you should betrained on its proper handling and storage. A regulated,marked area should be established where this chemical ishandled, used, or stored in compliance with OSHAStandard 1910.1045. Chromium chloride must be stored toavoid contact with water since violent reactions occur,releasing poisonous materials including chromic acid,hydrogen chloride, chromic chloride, and chlorine. Store intightly closed containers in a cool, well-ventilated areaaway from flammable and combustible materials, ammonia, alcohol and turpentine, and other incompatible materials listed above.
Shipping
UN1758 Chromium oxychloride, Hazard class: 8; Labels: 8-Corrosive material.
Purification Methods
Purify it by distillation under reduced pressure. It hydrolyses violently with H2O and is a powerful oxidant which explodes with P, and ignites in contact with S, NH3, EtOH and many organic compounds. TOXIC.
Incompatibilities
Contact with water is violent and forms hydrochloric and chromic acids, and chlorine gas. A powerful oxidizer. Reacts violently with acetone, alcohol, ammonia, ether, fuels, organic solvents, moist phosphorus, phosphorus trichloride; sodium azide; sulfur, reducing agents; turpentine. Contact with nonmetal halides, such as disulfur dichloride, phosphorus trichloride; and phosphorus tribromide; nonmetal hydrides, such as hydrogen sulfide; hydrogen phosphide, and urea, causes a danger fire and explosion hazard
Chromyl chloride Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21667 | 55 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 | linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-87569265 +86-15319487004 | 1015@dideu.com | China | 2132 | 58 |
| Antai Fine Chemical Technology Co.,Limited | 18503026267 | info@antaichem.com | CHINA | 9641 | 58 |
| Alfa Chemistry | info@alfa-chemistry.com | United States | 2344 | 58 | |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 | 1026@dideu.com | China | 8672 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 | sales@aladdinsci.com | United States | 52927 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 | sale@mainchem.com | China | 32360 | 55 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 | market03@meryer.com | China | 40240 | 62 |
| Alfa Aesar | 400-6106006 | saleschina@alfa-asia.com | China | 30132 | 84 |
View Lastest Price from Chromyl chloride manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2020-02-26 | Chromyl chloride
14977-61-8
|
US $0.00-0.00 / Kg | 1KG | 99.0%+ | 100 tons | Shaanxi Dideu Medichem Co. Ltd |
-

- Chromyl chloride
14977-61-8
- US $0.00-0.00 / Kg
- 99.0%+
- Shaanxi Dideu Medichem Co. Ltd