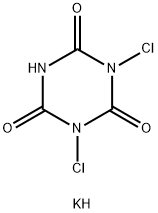
troclosene potassium
- CAS No.
- 2244-21-5
- Chemical Name:
- troclosene potassium
- Synonyms
- ACL-59;Fluonon;Nsc251767;Neochlor 59;troclosene potassium;POTASSIUM TROCLOSENE;POTASSIUMDICHLORO-S-TRIAZINETRIONE;Dichloroisocyanuric acid, potassium salt;Isocyanuric acid, dichloro-, potassium salt;Dichlor-S-triazin-2,4,6(1H,3H,5H)trione potassium
- CBNumber:
- CB7896545
- Molecular Formula:
- C3Cl2KN3O3
- Molecular Weight:
- 236.055
- MDL Number:
- MFCD00865980
- MOL File:
- 2244-21-5.mol
| Melting point | 250°C (rough estimate) |
|---|---|
| Indirect Additives used in Food Contact Substances | POTASSIUM DICHLOROISOCYANURATE |
| EWG's Food Scores | 1 |
| FDA UNII | 8A85D111P0 |
| EPA Substance Registry System | Potassium dichloro-s-triazinetrione (2244-21-5) |
troclosene potassium Chemical Properties,Uses,Production
Chemical Properties
Potassium dichloroisocyanurate is a white crys- talline solid. Chlorine odor.
Chemical Properties
White, slightly hygroscopic, crystalline powder or granules. Active ingredient 59% available chlorine; decomposes at 240C.
Uses
Useful source of available Cl in solid bleach and detergent formulations.
Definition
A cyclic com- pound.
General Description
White solid with an odor of chlorine. Mixes with water.
Air & Water Reactions
Water soluble. troclosene potassium may vigorously react with water releasing chlorine gas. Material containing less than 39% available chlorine will undergo reactions as described herein, but may take longer to initiate, and the resulting reaction may not be as vigorous [AAR 1992].
Reactivity Profile
Contact with ammonium compounds or hydrated salts can cause a very vigorous reaction. Prolonged exposure to heat /fire may result in the vigorous decomposition of the material with the rupture of its containers, troclosene potassium will accelerate the burning of combustible materials [AAR 1991]. Chlorine plus alcohols would yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites [NFPA 491 M. 1991].
Hazard
Moderately toxic by ingestion. Dangerous fire risk in contact with organic materials. A strong oxidizing agent.
Health Hazard
Dust causes sneezing and coughing; is moderately irritating to the eyes and causes itching and redness of skin. Ingestion causes burns of mouth and stomach.
Safety Profile
Moderately toxic by ingestion, causing ulceration or bleeding from stomach. A skin and severe eye irritant. Causes emaciation, weakness, lethargy, darrhea, weight loss. Autopsy indicates gastrointestinal tract irritation, tissue edema, liver and hdney congestion. A powerful oxidizer. When heated to decomposition it emits very toxic fumes of K2O, Cl-, and NOx.
Potential Exposure
Potassium dichloroisocyanurate is used in household bleaches, dishwashing compounds and detergents.
Shipping
UN2465 Dichloroisocyanuric acid, dry or Dichloroisocyanuric acid salts, Hazard Class: 5.1; Labels: 5.1-Oxidizer.
Incompatibilities
A strong oxidizer; violent reaction with reducing agents; combustibles, organics; easily chlorinated or oxidized materials; ammonia, urea, other nitrogen com- pounds; calcium hypochloride; other alkalies and moisture. Contact with ammonium compounds or hydrated salts can cause a very vigorous reaction. Prolonged exposure to heat/ fire may result in the vigorous decomposition of the mate- rial with the rupture of its containers, it will accelerate the burning of combustible materials. Chlorine plus alcohols would yield alkyl hypochlorites. They decompose in the cold and explode on exposure to sunlight or heat. Tertiary hypochlorites are less unstable than secondary or primary hypochlorites .
troclosene potassium Preparation Products And Raw materials
Raw materials
Preparation Products
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 | linda@hubeijusheng.com | CHINA | 28180 | 58 |
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 | 1046@dideu.com | China | 10008 | 58 |
| TargetMol Chemicals Inc. | 4008200310 | marketing@tsbiochem.com | China | 24131 | 58 |
| Supplier | Advantage |
|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 58 |
| TargetMol Chemicals Inc. | 58 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 58 |
| TargetMol Chemicals Inc. | 58 |