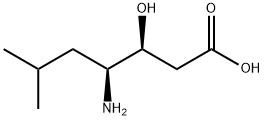
STATINE
- CAS No.
- 49642-07-1
- Chemical Name:
- STATINE
- Synonyms
- STATIN;STATINE;H-STA-OH;S)-Statine;LEU-STATINE;4S)-Statine;(S,S)-Statine;H-STA(3S,4S)-OH;(3S,4S)-STATINE;H-(3S,4S)-STA-OH
- CBNumber:
- CB9272763
- Molecular Formula:
- C8H17NO3
- Molecular Weight:
- 175.23
- MDL Number:
- MFCD00037277
- MOL File:
- 49642-07-1.mol
- MSDS File:
- SDS
| Melting point | 209 °C |
|---|---|
| alpha | D15 -20° (c = 0.64 in water) |
| Boiling point | 306.53°C (rough estimate) |
| Density | 1.1233 (rough estimate) |
| refractive index | 1.4476 (estimate) |
| storage temp. | 2-8°C |
| solubility | 0.5 M HCl: 50 mg/mL, clear, very faintly yellow |
| pka | 3.97±0.10(Predicted) |
| Merck | 13,8879 |
| NCI Dictionary of Cancer Terms | statin |
| FDA UNII | YTC77XR1EK |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H319-H315-H335 |
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313P-P264-P280-P302+P352-P321-P332+P313-P362 |
| Hazard Codes | Xi |
| Risk Statements | 36/37/38 |
| Safety Statements | 26-36 |
| WGK Germany | 3 |
STATINE price More Price(1)
STATINE Chemical Properties,Uses,Production
Chemical Properties
white powder
Mechanism of action
The statin family of six closely related hypocholesterolemic
drugs are all potent competitive inhibitors of
the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase
(HMG CoA reductase), the rate-limiting enzyme
in cholesterol biosynthesis.The liver is their target
organ, and decreased hepatic cholesterol synthesis ultimately
leads to increased removal of LDL particles from
the circulation. As a consequence, all other hypocholesterolemic
drugs have been relegated to secondary status.
Clinical trials with lovastatin (Mevacor), simvastatin
(Zocor) and pravastatin (Pravachol) provided much of
the evidence supporting the observation that lowering of blood cholesterol lowers the risk of CHD.
Reductions in CHD risk appear to be due to multiple
consequences of inhibiting the cholesterol synthesis
pathway. Drug-induced inhibition of hepatic cholesterol
synthesis leads to lowering of liver cholesterol concentrations
and feedback up-regulation at the gene level of
both HMG CoA reductase and the LDL receptor
(mechanisms IV and VII in Fig. 23.2). As long as the
statin is present at adequate concentration in the liver,
the extra HMG CoA reductase activity is not expressed.
However, the increased hepatic LDL receptor
protein results in increased rates of removal of LDL
particles from the circulation by the liver, lowering of blood LDL-cholesterol levels, slowing of atherosclerosis,
and decreased risk of heart attack.
The reduced risk of CHD achieved with the statins
may also be due to drug actions independent of lowering
blood cholesterol. Many important molecules besides
cholesterol are generated by intermediates in the
complex cholesterol synthesis pathway. These include
the isoprenes geranylgeranyl and farnesyl, which are covalently
attached to some proteins (isoprenylation) and
target them to membranes where they function.The re-ported capacities of statins to inhibit proliferation of arterial
wall smooth muscle cells and to improve endothelial
cell functions may be due to inhibited protein
isoprenylation in these cells secondary to HMG CoA
reductase inhibition.
Clinical Use
With the possible exception of atorvastatin, the
statins are used to lower LDL cholesterol in familial or
polygenic ( multifactorial) hypercholesterolemia (type
IIa) and in combination with triglyceride-lowering
drugs to treat combined hyperlipidemia (type IIb) when
both LDL and VLDL (very low density lipoproteins)
are elevated. However, the statins probably
should not be given with the fibrates (triglyceridelowering
drugs, discussed later), since this combination
may greatly increase statin toxicity. Atorvastatin, the
most potent of the available statins, has also
been shown to lower blood triglycerides significantly.
This effect may be due to decreasing hepatic cholesterol
and cholesterol ester levels to such an extent that
hepatic formation of VLDL is impaired.The statins also
have been claimed to reduce blood cholesterol levels
modestly in some patients with homozygous familial hypercholesterolemia,
a condition often fatal in childhood
or in early adulthood.
The statins may lower the risk of CHD by decreasing
inflammation, an important component of atherogenesis.
Lovastatin decreased elevated plasma levels of Creactive
protein, a marker for cellular inflammation, and
acute coronary events in patients with relatively low
plasma cholesterol levels. Recent studies also suggest
that use of statins may decrease the risk of stroke, dementia,
and Alzheimer’s disease and may improve bone density in postmenopausal women. These broad actions
may be related to the hypocholesterolemic, antiproliferative,
antiinflammatory, or antioxidant properties of the
statins or some combination of these properties.
Side effects
The statins generally appear to be well tolerated, with muscle pain and liver dysfunction seen in 1 to 2% of patients. However, the consequences of 20 to 30 years of continuous use are unknown. This fact has been dramatically reinforced by the recent recognition of a potentially fatal consequence of statin use. A relatively common side effect of the statins (perhaps 1% of patients) is myositis, that is, inflammation of skeletal muscle accompanied by pain, weakness, and high levels of serum creatine kinase. Rhabdomyolysis, i.e., disintegration of muscle with urinary excretion of myoglobin and kidney damage, was considered to be a rare and extreme toxic outcome. However, cerivastatin (Baycol) has now been withdrawn from the market by its manufacturer (Bayer) because of 31 deaths linked to fatal rhabdomyolysis. The risk of muscle damage is said to increase with simultaneous use of the triglyceride-lowering fibrates. Pravastatin may be less toxic than other statins because it does not readily penetrate extrahepatic cells and may be more confined to the liver after oral dosage.
STATINE Preparation Products And Raw materials
STATINE Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| TargetMol Chemicals Inc. | +1-781-999-5354 +1-00000000000 | marketing@targetmol.com | United States | 19892 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 15093356674; | laboratory@coreychem.com | China | 30255 | 58 |
| BOC Sciences | +16314854226 | inquiry@bocsci.com | United States | 19743 | 58 |
| Nextpeptide Inc | +86-0571-81612335 +8613336028439 | sales@nextpeptide.com | China | 19915 | 58 |
| LEAPCHEM CO., LTD. | +86-852-30606658 | market18@leapchem.com | China | 43348 | 58 |
| Beijing Ouhe Technology Co., Ltd | 010-82967028 13552068683 | 2355560935@qq.com | China | 12426 | 60 |
| Shanghai Hanhong Scientific Co.,Ltd. | 021-54306202 13764082696; | info@hanhongsci.com | China | 42982 | 64 |
| Chemsky (shanghai) International Co.,Ltd | 021-50135380 | shchemsky@sina.com | China | 15421 | 60 |
| SHANGHAI FORTUNE CHEMICAL TECHNOLOGY CO., LTD | 13816107857 | sales@fortunechem-sh.com | China | 289 | 58 |
| MedChemexpress LLC | 021-58955995 | sales@medchemexpress.cn | United States | 4863 | 58 |
49642-07-1(STATINE)Related Search:
1of4