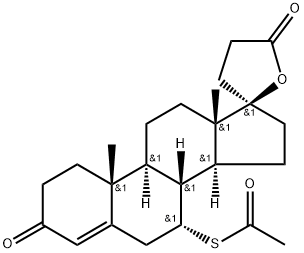
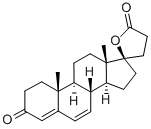
Spironolactone
- CAS No.
- 52-01-7
- Chemical Name:
- Spironolactone
- Synonyms
- Spirolactone;Osyrol;Dira;ALDACTONE;Spironolac;Altex;Osiren;sc9420;Acelat;Aldace
- CBNumber:
- CB9743463
- Molecular Formula:
- C24H32O4S
- Molecular Weight:
- 416.58
- MDL Number:
- MFCD00082250
- MOL File:
- 52-01-7.mol
| Melting point | 207-208 °C (lit.) |
|---|---|
| alpha | -37 º (c=1, CHCl3) |
| Boiling point | 504.87°C (rough estimate) |
| Density | 1.1061 (rough estimate) |
| refractive index | -36 ° (C=1, CHCl3) |
| storage temp. | 2-8°C |
| solubility | Practically insoluble in water, soluble in ethanol (96 per cent). |
| form | Powder |
| color | White to yellow-white |
| Water Solubility | practically insoluble |
| Merck | 14,8760 |
| BCS Class | 2,4,3 |
| LogP | 2.780 |
| CAS DataBase Reference | 52-01-7(CAS DataBase Reference) |
| FDA UNII | 27O7W4T232 |
| IARC | 3 (Vol. Sup 7, 79) 2001 |
| NIST Chemistry Reference | Spironolactone(52-01-7) |
| Proposition 65 List | Spironolactone |
| NCI Drug Dictionary | Aldactone |
| ATC code | C03DA01 |
| EPA Substance Registry System | Spironolactone (52-01-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H360 | |||||||||
| Precautionary statements | P201-P280-P308+P313 | |||||||||
| Hazard Codes | T,Xn,Xi | |||||||||
| Risk Statements | 60-40-36/37/38 | |||||||||
| Safety Statements | 53-22-36/37/39-45-36-26 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TU4725000 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 29321900 | |||||||||
| Toxicity | LD50 in rats, mice, rabbits (mg/kg): 790, 360, 870 i.p. (IARC, 1980) | |||||||||
| NFPA 704 |
|
Spironolactone price More Price(37)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich | S3378 | Spironolactone 97.0-103.0% | 52-01-7 | 1g | $84.7 | 2024-03-01 | Buy |
| Sigma-Aldrich | S3378 | Spironolactone 97.0-103.0% | 52-01-7 | 5G | $294 | 2024-03-01 | Buy |
| Sigma-Aldrich | S1200000 | Spironolactone European Pharmacopoeia (EP) Reference Standard | 52-01-7 | S1200000 | $220 | 2024-03-01 | Buy |
| Sigma-Aldrich | PHR2886 | Spironolactone pharmaceutical secondary standard, certified reference material | 52-01-7 | 200MG | $173 | 2024-03-01 | Buy |
| Sigma-Aldrich | Y0001295 | Spironolactone for system suitability European Pharmacopoeia (EP) Reference Standard | 52-01-7 | Y0001295 | $151 | 2023-06-20 | Buy |
Spironolactone Chemical Properties,Uses,Production
Diuretics
Spironolactone is a kind of weak potassium-sparing diuretics, and is fine white or white-like crystalline powder with slightly bitter taste. It is odorless or with slight mercaptan odor. It is highly soluble in chloroform but insoluble in water and soluble in ethanol, and easily soluble in benzene or ethyl acetate. It has a similar chemical structure as aldosterone and can compete with aldosterone for the aldosterone receptors in the cytoplasm of the distal convoluted tubule and collecting tube duct cells, affecting the binding of the aldosterone to its receptor, thus preventing the synthesis of aldosterone-induced protein and further inhibiting the exchange between K+ and Na+ with reduced potassium excretion and plays potassium-sparing diuretic effect.
The strength of the diuretic effect of spironolactone is related to the in vivo secretion of aldosterone with increased secretion also causing stronger effect while lower secretion causing weaker effects. It has no diuretic effect on patients without aldosterone secretion.
Because the drug only acts on the distal convoluted tubule and collecting duct while having no effect on the other segments of tubular, it only has a weak diuretic effect with slow onset but long duration time. It is mainly used for treating refractory edema accompanied with higher amount of aldosterone such as cirrhosis, congestive heart failure, and nephrotic syndrome with excellent efficacy in treating the patients with hyperaldosteronism caused by the lack of sodium in the congestive heart failure. Single drug administration usually only give a poor diuretic and therefore, it is often used in combination with hydrochlorothiazide or dihydrochlorothiazide for enhancing the effect. In addition, it can also be applied to the treatment of primary hyperaldosteronism and hypertension. Patients of hyperkalemia or renal failure should be disabled.
This product has a rapid and irregular absorption rate after oral administration with the absorption rate being relate to the spironolactone particle size. According to general process, the bioavailability is only about 5% while the absorption rate can reach 90% when made into fine particles with the time for the plasma concentration reaching the peak with approximately 3 hours. It has rapid metabolism rate in vivo with its major metabolites being canrenone which can exert the pharmacological activity and other metabolites also being gradually converted into canrenone. The serum metabolites level will reach peak at 2 to 4 hours after single dose of oral administration. The concentration of metabolites will decrease rapidly within 12 hours with slow decline in 12 to 96 hours. After oral administration of multiple doses, the half life of the canrenone metabolite was 13 to 24 hours. The half-life of spironolactone is only about 10 minutes. The plasma protein binding rate of canrenone is about 98%. Its metabolites can be secreted through the bile and urine. The canrenone metabolism includes enterohepatic circulation. It can penetrate through the placental barrier and enter into breast milk. It also has effects of inhibiting estrogen and androgen synthesis.
Indications
Spironolactone is a kind of competitive aldosterone inhibitors with the drug itself being not active. Its diuretic effect is through its competitive antagonism against endogenous aldosterone, belonging to potassium-sparing diuretics.
1. edema disease, being used in combination other diuretics for the treatment of congestive heart failure, liver cirrhosis, renal edema, edema disease; the purpose in to correct the increased secondary aldosterone secretion associated with those above diseases and fight against the potassium excretion of other diuretics; it can also used in the treatment of idiopathic edema.
2. Hypertension, as the auxiliary treatment drug of high blood pressure.
3. For primary hyperaldosteronism, spironolactone can be used to diagnose and treat the disease.
4. For the prevention of hypokalemia; being used in combination with thiazide diuretics to enhance the diuretic effect and prevent hypokalemia.
The above information is edited by the Chemicalbook of Dai Xiongfeng.
Uses
It can be used as a kind of inefficient diuretic drugs.
It is a synthetic 17-lactone steroid which is a kind of renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat ascites in patients with liver disease, low-renin hypertension and hypokalemia.
The active metabolite of lactone (spironolactone); It can be used for the Inhibition of aldosterone biosynthesis. It can also be used as the blocker of the Quabain effect.
Category
toxic substances
Toxicity grading
highly toxic
Acute toxicity
intraperitoneal-rat LD50: 277 mg/kg; intraperitoneal port-Mouse LD50: 260 mg/kg
Flammability and hazardous characteristics
easily flammable with combustion generating toxic fumes of sulfur oxides
Storage characteristics
Treasury: ventilation, low-temperature and dry; store it separately from food raw materials
Extinguishing agent
Dry powder foam, sand, carbon dioxide, spray water
Chemical Properties
White to Off White Solid
Originator
Aldactone,Searle,US,1959
Uses
diuretic, aldosterone antagonist
Uses
Spironolactone, an aldosterone-, and competitive androgen-receptor-antagonist and 5-alpharductase- inhibitor, indicated for the treatment of androgen dependent hirsutism, ideally in doses of 50 to 200 mg per day accompanying the intake of oral contraceptives with the same seven day break in between. Side effects concerning the length of the menstrual cycle, the increase of blood pressure or potassium levels may occur. Spironolactone is the number one drug in the treatment of hirsutism in the US (Farquhar et al., 2003). In other countries the prescription of spironolactone for the treatment of hirsutism may be off-label.
Uses
It is a synthetic 17-lactone steroid which is a renal competitive aldosterone antagonist in a class of pharmaceuticals called potassium-sparing diuretics, used primarily to treat ascites in patients with liver disease, low-renin hypertension, hypokalemia
Definition
ChEBI: A steroid lactone that is 17alpha-pregn-4-ene-21,17-carbolactone substituted by an oxo group at position 3 and an alpha-acetylsulfanyl group at position 7.
Indications
Spironolactone (Aldactone) is a compound originally developed as a mineralocorticoid antagonist and is used as a diuretic and antihypertensive agent. However, at high doses spironolactone binds to the androgen receptor. In clinical practice it is a weak androgen antagonist used to treat hirsutism in women by blocking testosterone binding to androgen receptors in hair follicles. Use of spironolactone in women for the treatment of hirsutism or male pattern baldness can result in elevated serum potassium levels; these levels should be checked within 1 month of starting the medication.
Manufacturing Process
A mixture of approximately 11 parts of 17α-(2-carboxyethyl)-17β- hydroxyandrosta-4,6-dien-3-one lactone and 10 parts of thioacetic acid is heated at 85° to 95°C for ? hour. Excess thioacetic acid is removed by vacuum distillation at this point, and the residue is twice recrystallized from methanol, affording 7α-acetylthio-17α-(2-carboxyethyl)-17β-hydroxyandrost- 4-en-3-one lactone, melting at approximately 134° to 135°C. Heated above this melting point, the product solidifies and melts again at approximately 201° to 202°C (with decomposition).
brand name
Aldactone (Searle);Airolactone;Aldactide 25;Aldactone-a;Aldazida;Aldonorm;Aldospirone;Alpamed;Altexide;Aporasnon;Carditan;Crk 635;Ct-spiro;Digi-aldopur;Dilakton;Hexalacton;Hokulaton;Hokuraton;Hydrospiron;Idrolattone;Lacilactone;Laralmin;Lasitone;Loractone;Mf 218d;Noidouble;Novospiroton;Novospirozine;Novosprioton;Novospriozine;Penantin;Pirolacton;Pirolcaton;Plarenil;Practon 50;Raudazida;Risicordin;Rolactone microfine;Sali-spiroctan;Sas 1060;Servilactone;Spiractin;Spiridazide;Spirix;Spiro comp;Spiro50-d;Spirodigital;Spiro-f;Spironomocompren;Spironone;Spironothiazide;Spiropal;Spirostada;Spirotone;Suprapuren;Synureticum;Tensoflex;Urosonine;Xenalone;Xeualon.
Therapeutic Function
Diuretic
World Health Organization (WHO)
Spironolactone, an aldosterone antagonist, has been widely used for over 25 years in the treatment of hypertension and in the management of refractive oedema. Evidence that long-term administration of high doses are tumorigenic in the rat has recently led to restriction of its use by some national regulatory authorities although the significance of this finding with respect to clinical use is not certain. In 1987 spironolactone was transferred from the main list to the complementary list of the WHO Model List of Essential Drugs. (See also WHO comments for canrenone and potassium canrenoate). (Reference: (WHODI) WHO Drug Information, 2(1), , 1988)
Biological Functions
Spironolactone (Aldactone) is structurally related to aldosterone and acts as a competitive inhibitor to prevent the binding of aldosterone to its specific cellular binding protein. Spironolactone thus blocks the hormone-induced stimulation of protein synthesis necessary for Na+ reabsorption and K+ secretion. Spironolactone, in the presence of circulating aldosterone, promotes a modest increase in Na+ excretion associated with a decrease in K+ elimination. The observations that spironolactone is ineffective in adrenalectomized patients and that the actions of spironolactone can be reversed by raising circulating al-dosterone blood levels (surmountable antagonism) support the conclusion that spironolactone acts by competitive inhibition of the binding of aldosterone with receptor sites in the target tissue. Spironolactone acts only when mineralocorticoids are present.
General Description
Spironolactone, 7α-(acetylthio)-17α-hydroxy-3-oxopregn-4-ene-3-one-21-carboxylic acidγ-lactone (Aldactone) is an aldosterone antagonist of greatmedical importance because of its diuretic activity.
Hazard
Questionable carcinogen.
Biological Activity
Competitive mineralocorticoid (aldosterone) receptor antagonist that exhibits antihypertensive activity in vivo . Also displays antiandrogen activity and inhibits steroid hormone biosynthesis.
Biochem/physiol Actions
Spironolactone is a competitive aldosterone receptor antagonist. Used as potassium sparing diuretic.
Mechanism of action
Spironolactone is a potassium sparing diuretic that has a different mechanism of action than other drugs of this class. It is a competitive antagonist of aldosterone, and its action is most effective when the level of circulated aldosterone in the organism is high.
Pharmacology
Spironolactone (Aldactone) is the only diuretic that has
been shown in a double-blind multicenter prospective
clinical trial to improve survival in CHF. The addition
of spironolactone to digitalis and an angiotensinconverting
enzyme (ACE) inhibitor significantly improved
survival among patients with chronic severe
heart failure.
Spironolactone competitively inhibits the binding of
aldosterone to cytosolic mineralocorticoid receptors in
the epithelial cells in the late distal tubule and collecting duct of the kidney. Aldosterone enhances salt and
water retention at the expense of enhanced renal K
and H excretion. Spironolactone enhances diuresis by
blocking sodium and water retention while retaining
potassium. An obvious potential side effect is hyperkalemia,
which is aggravated by the potassium-retaining
properties of the ACE inhibitors. The likely concomitant
use of the loop diuretic furosemide, which depletes
K , dictates careful monitoring of serum potassium to
avoid life-threatening rhythm disturbances.
There is also evidence for the existence of mineralocorticoid
receptors on cardiac myocytes. This raises the
intriguing possibility that spironolactone could mediate
important direct effects on the myocardium in CHF.
Clinical Use
Spironolactone has been used clinically in the following
conditions:
1. Primary hyperaldosteronism. Used as an aid in
preparing patients with adrenal cortical tumors
for surgery.
2. Hypokalemia. Used in patients with low serum K+
resulting from diuretic therapy with other agents.
Its use should be restricted to patients who are unable
to supplement their dietary K+ intake or adequately
restrict their salt intake or who cannot
tolerate orally available KCl preparations.
3. Hypertension and congestive heart failure.
Although spironolactone may be useful in combination
with thiazides, the latter remain the
drugs of first choice. Fixed-dose combinations of
spironolactone and a particular thiazide (e.g.,
Aldactazide) generally offer no therapeutic advantage
over either component given separately
and tend to restrict the ability of the clinician to
determine the optimal dosage of each drug for a
particular patient.
4. Cirrhosis and nephrotic syndrome. Spironolactone
is a mild diuretic and may be useful in treating the
edema that occurs in these two clinical conditions,
that is, when excessive K+ loss is to be avoided.
Side effects
Serum electrolyte balance should be monitored periodically, since potentially fatal hyperkalemia may occur,especially in patients with impaired renal function or excessive K+ intake (including the K+ salts of coadministered drugs, e.g., potassium penicillin). Spironolactone can induce hyponatremia and in cirrhotic patients, metabolic acidosis.A variety of gastrointestinal disturbances may accompany spironolactone administration. These include diarrhea, gastritis, gastric bleeding, and peptic ulcers. Spironolactone is contraindicated in patients with peptic ulcers. Spironolactone may also cause elevated blood urea nitrogen, drowsiness, lethargy, ataxia, confusion, and headache. Gynecomastia and menstrual irregularity in males and females, respectively, can occur. Painful gynecomastia (directly related to dosage level and duration of therapy), which is generally reversible, may necessitate termination of therapy. Animal studies demonstrating tumorigenic potential support the clinical judgment that spironolactone alone or in combination should not be used for most patients who require diuretic therapy and its unnecessary use should be avoided.
Safety Profile
Poison by intraperitoneal route. Human reproductive effects by ingestion and possibly other routes: men, impotence and breast development; women, menstrual cycle changes or disorders, changes in the breasts and lactation. An experimental teratogen. Other experimental reproductive effects. Other human systemic effects by ingestion: agranulocytosis, kidney tubule damage, increased urine volume, and changes in blood sodium and calcium levels. Questionable carcinogen. When heated to decomposition it emits toxic fumes of SOx,. Used to treat hypertension, edema of congestive heart failure, cirrhosis, and kidney failure. 0
Synthesis
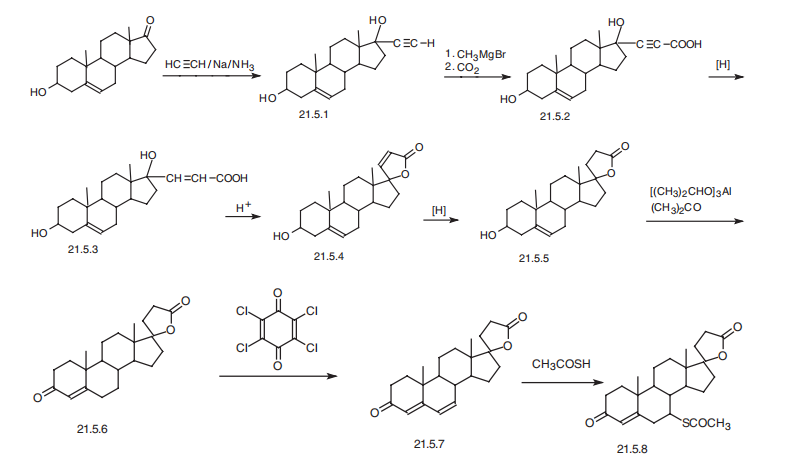
Spironolactone is the 7-acetate of the |?-lactone of 17-hydroxy-7-mercapto- 3-oxo-17-|á-pregn-4-ene-21-carboxylic acid (21.5.8). Spironolactone is synthesized industrially in two different ways from androstenolone?a3|?-hydroxy-5-androsten-17-one. According to the first method, androstenolone undergoes ethynylation by acetylene in a Normant reaction condition using sodium amide in liquid ammonia, which forms 17 |á-ethynyl-3|?-,17|?-dihydroxy-5-androstene (21.5.1). Subsequent reaction of this with methylmagnesiumbromide and then with carbon dioxide gives the corresponding propenal acid (21.5.2). Reduction of the triple bond in this product with hydrogen using a palladium on calcium carbonate catalyst forms the corresponding acrylic acid derivative (21.5.3), which is treated with acid without being isolated, which leads to cyclization into an unsaturated lactone derivative (21.5.4). The double bond is reduced by hydrogen, in this case using a palladium on carbon catalyst. The resulting lactone 21.5.5 undergoes oxidation in an Oppenauer reaction, giving an unsaturated keto-derivative?a4-androsten-3,17-dione (21.5.6). Further oxidation of the product (21.5.6) using chloroanyl gives dienone (21.5.7), which when reacted with thioacetic acid gives the desired spionolactone (21.5.8).
Veterinary Drugs and Treatments
Spironolactone may be used in patients with congestive heart failure who do not adequately respond to furosemide and ACE inhibitors, who develop hypokalemia on other diuretics, and are unwilling or unable to supplement with exogenous potassium sources. It may also be effective in treating ascites as it has less potential to increase ammonia levels than other diuretics.
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors or angiotensin-II antagonists:
enhanced hypotensive effect; risk of severe
hyperkalaemia.
Antibacterials: avoid with lymecycline.
Antidepressants: increased risk of postural
hypotension with tricyclics.
Antihypertensives: enhanced hypotensive effect;
increased risk of first dose hypotensive effect with
post-synaptic alpha-blockers.
Cardiac glycosides: increased digoxin concentration.
Ciclosporin: increased risk of hyperkalaemia.
Cytotoxics: avoid with mitotane; increased risk
of nephrotoxicity and ototoxicity with platinum
compounds.
Lithium: reduced lithium excretion.
NSAIDs: increased risk of hyperkalaemia (especially
with indometacin); increased risk of nephrotoxicity;
diuretic effect of spironolactone antagonised by
aspirin.
Potassium salts: increased risk of hyperkalaemia.
Tacrolimus: increased risk of hyperkalaemia.
Metabolism
Spironolactone is poorly absorbed after oral administration and has a delayed onset of action; it may take several days until a peak effect is produced. It has a somewhat slower onset of action than triamterene and amiloride (discussed later), but its natriuretic effect is modestly more pronounced, especially during long-term therapy. Spironolactone is rapidly and extensively metabolized, largely to the active metabolite canrenone. Canrenone and potassium canrenoate, its K+ salt, are available for clinical use in some countries outside the United States. Canrenone has a half-life of approximately 10 to 35 hours.The metabolites of spironolactone are excreted in both the urine and feces. New selective aldosterone receptor antagonists (SARA), such as eplerenone, have been developed but have not yet been introduced into clinical practice. Eplerenone and canrenone exhibit fewer steroidlike side effects (gynecomastia, hirsutism).
storage
Room temperature
Structure and conformation
Synthetic steroid that resembles aldosterone.
Spironolactone Preparation Products And Raw materials
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| APOLLO HEALTHCARE RESOURCES | +6596580999 | sales@apollo-healthcare.com.sg | Singapore | 400 | 58 |
| Jinan Qinmu Fine Chemical Co.,Ltd. | +8618660799346 | sales@qinmuchem.com | China | 1009 | 58 |
| Hebei Kangcang new material Technology Co., LTD | +8615713292910 | Nancy@kangcang.com.cn | China | 341 | 58 |
| Hebei Mojin Biotechnology Co., Ltd | +8613288715578 | sales@hbmojin.com | China | 12471 | 58 |
| Henan Bao Enluo International TradeCo.,LTD | +86-17331933971 +86-17331933971 | deasea125996@gmail.com | China | 2503 | 58 |
| Hangzhou Hyper Chemicals Limited | +86-0086-57187702781 +8613675893055 | info@hyper-chem.com | China | 103 | 58 |
| hebei hongtan Biotechnology Co., Ltd | +86-86-1913198-3935 +8617331935328 | sales03@chemcn.cn | China | 952 | 58 |
| Beijing Cooperate Pharmaceutical Co.,Ltd | 010-60279497 | sales01@cooperate-pharm.com | CHINA | 1811 | 55 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 | info@tianfuchem.com | China | 21689 | 55 |
| Hefei TNJ Chemical Industry Co.,Ltd. | +86-0551-65418679 +86-18949832763 | info@tnjchem.com | China | 2989 | 55 |
View Lastest Price from Spironolactone manufacturers
| Image | Update time | Product | Price | Min. Order | Purity | Supply Ability | Manufacturer | |
|---|---|---|---|---|---|---|---|---|
 |
2024-05-17 | Spironolactone
52-01-7
|
US $20.00 / kg | 1kg | 98% | 2000kg | hebei hongtan Biotechnology Co., Ltd | |
 |
2024-05-11 | Spironolactone
52-01-7
|
US $20.00-10.00 / kg | 1kg | 98% | 20 | Hebei Kangcang new material Technology Co., LTD | |
 |
2024-05-11 | Spironolactone
52-01-7
|
US $9.00-60.00 / g | 10g | 99% | 10 tons | Hebei Kangcang new material Technology Co., LTD |
-

- Spironolactone
52-01-7
- US $20.00 / kg
- 98%
- hebei hongtan Biotechnology Co., Ltd
-

- Spironolactone
52-01-7
- US $20.00-10.00 / kg
- 98%
- Hebei Kangcang new material Technology Co., LTD
-

- Spironolactone
52-01-7
- US $9.00-60.00 / g
- 99%
- Hebei Kangcang new material Technology Co., LTD
52-01-7(Spironolactone)Related Search:
1of4