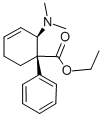
tilidine
- CAS No.
- 20380-58-9
- Chemical Name:
- tilidine
- Synonyms
- tilidine;dl-trans-Tilidine;WDEFBBTXULIOBB-WBVHZDCISA-N;Ethyl (1S,2R)-2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate;rel-2α*-(Dimethylamino)-1-phenyl-3-cyclohexene-1β*-carboxylic acid ethyl ester;rel-2β*-(Dimethylamino)-1β*-phenyl-3-cyclohexene-1α*-carboxylic acid ethyl ester
- CBNumber:
- CB9895012
- Molecular Formula:
- C17H23NO2
- Molecular Weight:
- 273.374
- MDL Number:
- MOL File:
- 20380-58-9.mol
| Boiling point | bp0.01 95.5-96° |
|---|---|
| FDA UNII | GY33N31E9Y |
| ATC code | N02AX01 |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|---|---|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264-P270-P301+P312-P330-P501 |
tilidine Chemical Properties,Uses,Production
Originator
Valoron,Goedecke,W. Germany,1970
Uses
Tilidine is an opioid used primarily as a painkiller it inhibits the enzyme adenylyl cyclase.
Definition
ChEBI: Dextilidine is an ethyl 2-(dimethylamino)-1-phenylcyclohex-3-ene-1-carboxylate that has S configuration at the carbon bearing the phenyl group and R configuration at the carbon bearing the dimethylamino group. The opioid analgesic tilidine is the racemate comprising equimolar amounts of dextilidine and its enantiomer, ent-dextilidine. A prodrug, tilidine is converted by the liver to the active analgesic, nortilidine; virtually all of the opioid activity resides in the (1S,2R) isomer (i.e. the isomer derived from dextilidine). It has a role as an opioid analgesic and a prodrug. It is an enantiomer of an ent-dextilidine.
Manufacturing Process
In a first step, dimethylamine is reacted with crotonaldehyde to give 1-
(dimethylamino)-1,3-butadiene.
A solution of 194 grams (2 mols) of fresh-distilled 1-(dimethylamino)-1,3-
butadiene is combined at room temperature in a 1 liter round-bottom flask
with 352 grams (2 mols) atropic acid ethyl ester. After being stirred for about
10 minutes, the reaction mixture gradually becomes exothermic. By cooling
with ice water, the contents of the flask are kept at a temperature of 40° to
60°C. After the reaction has ceased, the mixture is kept overnight (about 8 to 24 hours) at room temperature. The next day the viscous product is dissolved
in 10 liters of ether and precipitated with ethereal hydrogen chloride forming
the corresponding hydrochloride. By fractional crystallization from ethyl
acetate/methyl ethyl ketone (10:1), an almost complete separation of the
isomeric cis/trans isomers (I) and (II) is achieved. The separation can be
carried out very easily due to the low solubility of the 1 1/2-hydrate of (I).
Therefore, during the crystallization a sufficient quantity of water for the
formation of the 1 1/2-hydrate of (I) is added to the mixture of solvents,
whereby (I) readily precipitates.
Isomer (I): 4-phenyl-3-cis-dimethylamino-4-cis-carbethoxy-?1-
cyclohexenehydrochloride, [ethyl-cis-3-(dimethylamino)-4-phenyl-1-
cyclohexene-4-carboxylate hydrochloride] , MP 84°C (the free base boils at
97.5° to 98°C at 0.01 mm pressure), 64.4% yield.
Isomer (II): 4-phenyl-3-trans-dimethylamino-4-trans-carbethoxy-?1-
cyclohexenehydrochloride, [ethyl-trans-3-(dimethylamino)-4-phenyl-1-
cyclohexene-4-carboxylate hydrochloride], MP 159°C (the free base boils at
95.5° to 96°C at 0.01 mm pressure), 22.2% yield.
Therapeutic Function
Analgesic