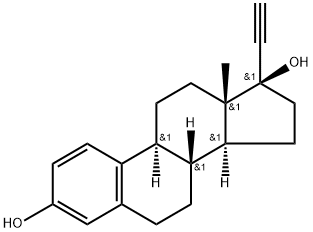
Ethinyl Estradiol
- CAS No.
- 57-63-6
- Chemical Name:
- Ethinyl Estradiol
- Synonyms
- ETHINYL ESTRADIOL;ETHYNYLESTRADIOL;17α-Ethynylestradiol;Estradio;17a-ethinylestradiol;17ALPHA-ETHYNYLESTRADIOL;(8R,9S,13S,14S,17R)-17-ethynyl-13-Methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol;eed;17-Ethinylestradiol;LYNORAL
- CBNumber:
- CB1350377
- Molecular Formula:
- C20H24O2
- Molecular Weight:
- 296.41
- MOL File:
- 57-63-6.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/4/3 17:07:09
| Melting point | 182-183 °C(lit.) |
|---|---|
| Boiling point | 378°C (rough estimate) |
| Density | 1.0944 (rough estimate) |
| refractive index | -30 ° (C=0.4, Pyridine) |
| Flash point | 9℃ |
| storage temp. | room temp |
| solubility | ethanol: 50 mg/mL, clear, slightly yellow |
| pka | pKa 10.32 (Uncertain) |
| color | White to Light yellow to Light orange |
| Merck | 14,3734 |
| BRN | 2419975 |
| BCS Class | 3/1 |
| InChIKey | BFPYWIDHMRZLRN-SLHNCBLASA-N |
| CAS DataBase Reference | 57-63-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Ethinyl estradiol(57-63-6) |
| EPA Substance Registry System | Ethinyl estradiol (57-63-6) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |    GHS07,GHS08,GHS09 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Danger | |||||||||
| Hazard statements | H302-H350-H410 | |||||||||
| Precautionary statements | P202-P264-P270-P273-P301+P312-P308+P313 | |||||||||
| Hazard Codes | T,F | |||||||||
| Risk Statements | 45-22-39/23/24/25-23/24/25-11 | |||||||||
| Safety Statements | 53-36/37/39-45-36/37-16 | |||||||||
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | RC8925000 | |||||||||
| F | 8 | |||||||||
| HS Code | 29372390 | |||||||||
| Toxicity | LD50 oral in rat: 960mg/kg | |||||||||
| NFPA 704 |
|
Ethinyl Estradiol price More Price(7)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | E4876 | 17α-Ethynylestradiol ≥98% | 57-63-6 | 100MG | ₹2262.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E4876 | 17α-Ethynylestradiol ≥98% | 57-63-6 | 1G | ₹7891.43 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1480 | Ethinyl Estradiol Pharmaceutical Secondary Standard; Certified Reference Material | 57-63-6 | 200MG | ₹11366.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E4876 | 17α-Ethynylestradiol ≥98% | 57-63-6 | 10G | ₹65339.7 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E-076 | 17α-Ethynylestradiol solution 1.0?mg/mL in methanol, ampule of 1?mL, certified reference material, Cerilliant? | 57-63-6 | 1ML | ₹11943.4 | 2022-06-14 | Buy |
Ethinyl Estradiol Chemical Properties,Uses,Production
Description
Estrogens direct the development of the female genotype in embryogenesis and at puberty. Estradiol is the major estrogen secreted by the premenopausal ovary. Ethynyl estradiol is a synthetic analog of 17β-estradiol . A USP-approved grade of ethynyl estradiol is often formulated in combination with a progestin such as norgestrel /levonorgestrel or desogestrel and provided for use as an oral contraceptive. Efficacy of oral administration of ethynyl estradiol is facilitated by the ethynyl substitution at the C-17 position, which inhibits first pass hepatic metabolism. Ethynyl estradiol is also rapidly and almost completely absorbed from the gastrointestinal tract.
Chemical Properties
Off-White to Light-Yellow Crystalline Powder
Uses
A metabolite of 17a-Ethynylestradiol
Definition
ChEBI: A 3-hydroxy steroid that is estradiol substituted by a ethynyl group at position 17. It is a xenoestrogen synthesized from estradiol and has been shown to exhibit high estrogenic potency on oral administration.
General Description
Fine white to creamy white powder. A synthetic steroid. Used in combination with progestogen as an oral contraceptive.
Air & Water Reactions
Air and light sensitive . Insoluble in water.
Reactivity Profile
Ethynyl estradiol may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to generate gaseous hydrogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition Ethynyl estradiol emits acrid smoke and fumes.
Fire Hazard
The flash point data for Ethynyl estradiol are not available. Ethynyl estradiol is probably combustible.
Mechanism of action
Synthetic estrogen with potent activity (inhibition of ovulation), widely used in oral contraceptives. Manufactured from natural estrogen, estrone, by reaction with potassium acetylide (HCRCK) in liquid ammonia. The synthetic 17α-ethynyl derivative of estradiol-17β. The 17α-ethynyl group increases the in vivo potency of estradiol- 17β by blocking the action of 17β-dehydrogenase, a major pathway of estradiol-17β metabolic inactivation. It is thus active orally and is among the most potent of the known estrogenic compounds.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, tumorigenic, and neoplastigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Human systemic effects by ingestion: glandular effects. An experimental teratogen. Experimental reproductive effects. Human mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also ESTRADIOL
Potential Exposure
The working environment may be contaminated during sex hormone manufacture, especially during the extraction and purification of natural steroid hormones; grinding of raw materials; handling of powdered products and recrystallization. Airborne particles of sex hormones may be absorbed through the skin, ingested or inhaled. Enteric absorption results in quick inactivation of sex hormones in the liver. The rate of inactivation is decreased for the oral, alkylated steroid hormones (methyl testosterone, anabolic steroids, etc.). Sex hormones may accumulate and reach relatively high levels even if their absorption is intermittent. Consequently, repeated absorption of small amounts may be detrimental to health. Intoxication by sex hormones may occur in almost all the exposed workers if preventive measures are not taken. The effect in the industrial sector is more successful than the agricultural one (chemical caponizing of cockerels by stilbestrol implants and incorporation of estrogens in feed for body weight gain promotion in beef cattle), where measures taken are summary and the number of cases of intoxication is consequently bigger
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
17--Ethynylestradiol forms a hemihydrate on recrystallising from MeOH/H2O. It dehydrates on melting and remelts on further heating at m 182-184o. The UV has max at 281nm ( 2040) in EtOH. Its solubility is 17% in EtOH, 25% in Et2O, 20% in Me2CO, 25% in dioxane and 5% in CHCl3. [Petit & Muller Bull Soc Chim Fr 121 1951.] The diacetyl derivative has m 143-144o (from MeOH) and [] D 20 +1o (c 1, CHCl3) [Mills et al. J Am Chem Soc 80 6118 1958]. [Beilstein 6 IV 6877.]
Incompatibilities
May react exothermically with reducing agents to generate flammable gaseous hydrogen. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or waste drugs and pharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator
Ethinyl Estradiol Preparation Products And Raw materials
Raw materials
1of2
chevron_rightPreparation Products
| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Gonane Pharma | +91-9819380043 +91-9819380043 | NaviMumbai, India | 192 | 58 | Inquiry |
| PANCHSHEEL ORGANICS LTD | +91-9821053955 +91-9324201019 | Mumbai, India | 82 | 58 | Inquiry |
| Lupin Ltd | +91-8019896181 +91-8019896181 | Maharashtra, India | 93 | 58 | Inquiry |
| Asg Biochem Pvt. Ltd | +91-9650436669 +91-9650436669 | New Delhi, India | 25 | 58 | Inquiry |
| Shakti Life Science | +91-9930420063 +91-9930420063 | Maharashtra, India | 28 | 58 | Inquiry |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | +91-2249700250 +91-9619320820 | Mumbai, India | 108 | 58 | Inquiry |
| Swati Spentose Pvt Ltd | +91-2261505232 +91-2261505232 | Maharashtra, India | 44 | 58 | Inquiry |
| Ralington Pharma | +91-7948911722 +91-9687771722 | Gujarat, India | 1350 | 58 | Inquiry |
| Orgamine Chemicals(I) Pvt Ltd | +91-9820080281 +91-9820080281 | Maharashtra, India | 451 | 58 | Inquiry |
| Symbiotec Pharma Lab Pvt Ltd | +91-7316676406 +91-7316676405 | Madhya Pradesh, India | 71 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| Gonane Pharma | 58 |
| PANCHSHEEL ORGANICS LTD | 58 |
| Lupin Ltd | 58 |
| Asg Biochem Pvt. Ltd | 58 |
| Shakti Life Science | 58 |
| Basil Drugs AND Pharmaceuticals Pvt Ltd | 58 |
| Swati Spentose Pvt Ltd | 58 |
| Ralington Pharma | 58 |
| Orgamine Chemicals(I) Pvt Ltd | 58 |
| Symbiotec Pharma Lab Pvt Ltd | 58 |
Related articles
- Levonorgestrel-Ethinyl Estradiol Oral: uses and side effects
- This combination hormone medication Levonorgestrel-Ethinyl Estradiol is used to prevent pregnancy.
- Mar 20,2024
57-63-6(Ethinyl Estradiol)Related Search:
1of4
chevron_right




