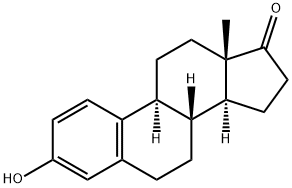
Estrone
- CAS No.
- 53-16-7
- Chemical Name:
- Estrone
- Synonyms
- Estron;200-164-5;1,3,5(10)-ESTRATRIEN-3-OL-17-ONE;Esterone;Hiestrone;OESTRONE;(8R,9S,13S,14S)-3-hydroxy-13-Methyl-7,8,9,11,12,13,15,16-octahydro-6H-cyclopenta[a]phenanthren-17(14H)-one;ESTROL;FOLLICULIN;Theelin
- CBNumber:
- CB5741416
- Molecular Formula:
- C18H22O2
- Molecular Weight:
- 270.37
- MOL File:
- 53-16-7.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/3/27 16:40:14
| Melting point | 258-260 °C(lit.) |
|---|---|
| Boiling point | 353.48°C (rough estimate) |
| alpha | 158 º (c=1, dioxane) |
| Density | 1.2360 |
| refractive index | 165 ° (C=1, Dioxane) |
| Flash point | 9℃ |
| storage temp. | room temp |
| solubility | Chloroform (Slightly), Dioxane (Slightly), Ethanol (Slightly), Methanol (Slightly) |
| pka | pKa 10.77±0.02(H2O)(Approximate) |
| form | Crystalline Powder or Crystals |
| color | White to almost white |
| Water Solubility | 0.03 g/L |
| Merck | 3708 |
| BRN | 1915077 |
| InChIKey | DNXHEGUUPJUMQT-CBZIJGRNSA-N |
| CAS DataBase Reference | 53-16-7(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Hydroxyestra-1,3,5(10)-trien-17-one(53-16-7) |
| EPA Substance Registry System | Estrone (53-16-7) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS08 |
|---|---|
| Signal word | Danger |
| Hazard statements | H351-H360FD-H362 |
| Precautionary statements | P202-P260-P263-P264-P270-P308+P313 |
| Hazard Codes | T,F |
| Risk Statements | 45-60-61-64-40-63-39/23/24/25-23/24/25-11 |
| Safety Statements | 53-45-36/37-16 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | KG8575000 |
| HS Code | 29335995 |
Estrone price More Price(11)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | E9750 | Estrone ≥99% | 53-16-7 | 500MG | ₹3117.6 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E9750 | Estrone ≥99% | 53-16-7 | 1G | ₹4589.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | PHR1535 | Estrone Pharmaceutical Secondary Standard; Certified Reference Material | 53-16-7 | 500MG | ₹13238.98 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E9750 | Estrone ≥99% | 53-16-7 | 5G | ₹17428.25 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | E9750 | Estrone ≥99% | 53-16-7 | 25G | ₹56019.38 | 2022-06-14 | Buy |
Estrone Chemical Properties,Uses,Production
Description
Estrone is one of the three naturally occurring estrogens, the others being estradiol and estriol. Estrone is synthesized from androstenedione by the aromatase enzyme system in the ovaries and placenta, and is also synthesized from estradiol by 17-hydroxy steroid dehydrogenase in the liver.Serum concentrations of estrone in premenopausal women fluctuate according to the menstrual cycle and becomes the most predominant estrogen in postmenopausal women.The binding affinities of estrone to the estrogen receptors α and β are approximately 60% and 37% relative to estradiol.
Chemical Properties
Estrone is an odorless white crystalline powder.
Estrone is supplied as a crystalline solid. A stock solution may be made by dissolving the estrone in an organic solvent purged with an inert gas. Estrone is soluble in organic solvents such as DMSO and dimethyl formamide (DMF). The solubility of estrone in these solvents is approximately 20 mg/ml.
Estrone is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, estrone should first be dissolved in DMF and then diluted with the aqueous buffer of choice. Estrone has a solubility of approximately 0.15 mg/ml in a1:5 solution of DMF:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day.
Uses
Estrone is a metabolite of 17β-Estradiol (E888000). During the metabolism, it is in rapid equilibrium with Estriol (E888960) and 17β-Estradiol (E888000) (1). Causes the feminization of male fish at human and animal waste sites (2). This compound is a contaminant of emerging concern (CECs). Drinking water contaminant candidate list 3 (CCL 3) compound as per United States Environmental Protection Agency (EPA), environmental, and food contaminants.
Definition
ChEBI: A 17-oxo steroid that is estra-1,3,5(10)-triene substituted by an hydroxy group at position 3 and an oxo group at position 17.
General Description
Estrone, 3-hydroxyestra-1,3,5(10)-trien-17-one, is less active than estradiol but more active than itsmetabolite, estriol. As the salt of its 3-sulfate ester, estroneis the primary ingredient in conjugated estrogens, USP, andesterified estrogens, USP. Although originally obtainedfrom the urine of pregnant mares (about 10 mg/L), estroneis now prepared synthetically. Estrone itself is not availablein commercial oral formulations, but can be obtained at compounding pharmacies as a topical formulation. Oleoylestrone,the C3 ester of estrone with oleic acid, is in phase IIclinical trials for the treatment of obesity. This acyl estronederivative reduces fat stores by a mechanism not involvingthe ER, although some of the oleoyl-estrone is hydrolyzedto estrone in vivo.
Hazard
A carcinogen (OSHA).
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, tumorigenic, and teratogenic data. A poison by intraperitoneal and subcutaneous routes. Human reproductive effects by implantation: spermatogenesis and impotence. Mutation data reported. A steroid drug for the treatment of menopause and ovariectomy symptoms. When heated to decomposition it emits acrid smoke and irritating fumes.
Potential Exposure
Synthesized from ergosterol. Used in combination with progestogen as an oral contraceptive.
Shipping
UN3249 Medicine, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Purify estrone by chromatography on silica gel, eluting with 2:1 hexane/EtOAc and recrystallising from EtOH or Et2O/EtOH. [Danishefsky & Cain J Am Chem Soc 98 4975 1976.] The acetate [901-93-9] crystallises from EtOH with m 125-127o. [Beilstein 8 III 1171.]
Incompatibilities
May react exothermically with reducing agents to generate flammable gaseous hydrogen. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, and epoxides.
Estrone Preparation Products And Raw materials
Raw materials
1of3
chevron_rightPreparation Products
1of2
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| Alfa Omega Pharma | +91-8050045945 +91-9972665399 | Maharashtra, India | 126 | 58 | Inquiry |
| Symbiotec Pharma Lab Pvt Ltd | +91-7316676406 +91-7316676405 | Madhya Pradesh, India | 71 | 58 | Inquiry |
| TCI Chemicals (India) Pvt. Ltd. | 1800 425 7889 | New Delhi, India | 6778 | 58 | Inquiry |
| CLEARSYNTH LABS LTD. | +91-22-45045900 | Hyderabad, India | 6351 | 58 | Inquiry |
| A.J Chemicals | 91-9810153283 | New Delhi, India | 6124 | 58 | Inquiry |
| Otto Chemie Pvt. Ltd. | +91 9820041841 | Mumbai, India | 5873 | 58 | Inquiry |
| Pharmaffiliates Analytics and Synthetics P. Ltd | +91-172-5066494 | Haryana, India | 6773 | 58 | Inquiry |
| Central Drug House(P) Ltd. | 91-11-49404040 | New Delhi, India | 6160 | 58 | Inquiry |
| SynZeal Research Pvt Ltd | +1 226-802-2078 | Gujarat, India | 6522 | 58 | Inquiry |
| SYMBIOTEC PHARMALAB PRIVATE LTD | +91-731-4200052 | New Delhi, India | 55 | 58 | Inquiry |
Related articles
- Synthesis and use of Estrone
- Estrone is a steroid hormone produced in the ovary, testis, and placenta; it has weak estrogenic activity.
- Jun 16,2022
- What is Estrone?
- Estrone, one of the major mammalian estrogens, is an aromatized C18 steroid with a 3-hydroxyl group and a 17-ketone. It is pro....
- Jan 18,2022
53-16-7(Estrone)Related Search:
1of4
chevron_right




