Sodium carbonate
- CAS No.
- 497-19-8
- Chemical Name:
- Sodium carbonate
- Synonyms
- SODIUM THIOSULPHATE;Disodium carbonate;ANHYDROUS SODIUM CARBONATE;Sodium carbonate anhydrous;ASH;Disodium salt;Sodium carbonate solution;Carbonic acid sodium salt;HYPO;Natriumcarbonat
- CBNumber:
- CB9853672
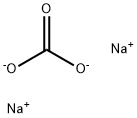
- Molecular Formula:
- CH2O3.2Na
- Molecular Weight:
- 105.99
- MOL File:
- 497-19-8.mol
- MSDS File:
- SDS
- Modify Date:
- 2024/5/18 8:51:57
| Melting point | 851 °C (lit.) |
|---|---|
| Boiling point | 1600°C |
| Density | 2.53 |
| refractive index | 1.535 |
| storage temp. | 15-25°C |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| Specific Gravity | 2.532 |
| color | White |
| PH | 10.52(1 mM solution);10.97(10 mM solution);11.26(100 mM solution); |
| Odor | at 100.00?%. odorless |
| pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) |
| Water Solubility | 22 g/100 mL (20 ºC) |
| Sensitive | Hygroscopic |
| λmax |
λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
| Merck | 14,8596 |
| BRN | 4154566 |
| Dielectric constant | 5.3(Ambient) |
| Stability | Stable. Incompatible with powdered alkaline earth metals, aluminium, organic nitro compounds, fluorine, alkali metals, nonmetallic oxides, concentrated sulfuric acid, oxides of phosphorus. |
| InChIKey | CDBYLPFSWZWCQE-UHFFFAOYSA-L |
| CAS DataBase Reference | 497-19-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Sodium carbonate(497-19-8) |
| EPA Substance Registry System | Sodium carbonate (497-19-8) |
SAFETY
Risk and Safety Statements
| Symbol(GHS) |  GHS07 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Signal word | Warning | |||||||||
| Hazard statements | H319 | |||||||||
| Precautionary statements | P264-P280-P305+P351+P338-P337+P313 | |||||||||
| Hazard Codes | Xn,Xi | |||||||||
| Risk Statements | 32-36-51/53-36/37/38-41-37/38 | |||||||||
| Safety Statements | 36/37-26-22-36-39 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | XN6476000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| HS Code | 28362000 | |||||||||
| Toxicity | LD50 orally in Rabbit: 4090 mg/kg | |||||||||
| NFPA 704 |
|
Sodium carbonate price More Price(119)
| Manufacturer | Product number | Product description | CAS number | Packaging | Price | Updated | Buy |
|---|---|---|---|---|---|---|---|
| Sigma-Aldrich(India) | S7795 | Sodium carbonate BioXtra, ≥99.0% | 497-19-8 | 500G | ₹9770 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S7795 | Sodium carbonate BioXtra, ≥99.0% | 497-19-8 | 1KG | ₹19127.78 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S2127 | Sodium carbonate ReagentPlus?, ≥99.5% | 497-19-8 | 1KG | ₹9439.4 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | S2127 | Sodium carbonate ReagentPlus?, ≥99.5% | 497-19-8 | 5KG | ₹26672.8 | 2022-06-14 | Buy |
| Sigma-Aldrich(India) | 900505 | Sodium carbonate anhydrous, free-flowing, Redi-Dri?, ReagentPlus?, ≥99.5% | 497-19-8 | 500G | ₹6397.58 | 2022-06-14 | Buy |
Sodium carbonate Chemical Properties,Uses,Production
Description
Sodium carbonate is known as soda ash or washing soda and is a heavily used inorganic compound. Approximately 45 million tons of soda ash are produced globally both naturally and synthetically. Soda ash is obtained naturally primarily from the mineral trona, but it can also be obtained from nahcolite (NaHCO3) and salt brine deposits. Trona is a freshwater sodium carbonate-bicarbonate evaporite, with the formula Na3CO3HCO3 .2H2O. The largest known deposit of trona is located in the Green River area of Wyoming, and other large deposits are found in Egypt’s Nile Valley and California’s Searles basin around the city of Trona. Soda ash is produced from mined trona by crushing and screening the ore and then heating it. Th is produces a soda ash mixed with impurities. Pure soda ash is obtained by dissolving the product and precipitating impurities combined with filtering processes.
Chemical Properties
Sodium carbonate, Na2C03, also known as soda or soda ash,is the most important of the industrial alkalis. It is a white or grayish-white, lumpy, water-soluble powder that loses its water of crystallization when heated. It decomposes at a temperature of about 852°C (1560°F). It exists in solution only. It is prepared by the combination of carbon dioxide and water.
Physical properties
Anhydrous sodium carbonate (soda ash, sal soda) is a white powder, which cakes and aggregates on exposure to air due to the formation of hydrates. The monohydrate, Na2CO3·H2O, is a white crystalline material, which is soluble in water and insoluble in alcohol; r.d. 2.532; loses water at 109°C; m.p. 851°C. The decahydrate, Na2CO3·10H2O (washing soda), is a translucent efluorescent crystalline solid; r.d. 1.44; loses water at 32–34°C to give the monohydrate; m.p. 851°C.
Occurrence
Ash is a tree found in regions of North America
History
Sodium carbonate, Na2CO3, has been used historically for making glass, soap, and gunpowder. Along with potassium carbonate, known as potash, sodium carbonate was the basis of the alkali industry, which was one of the first major chemical industries. Throughout history, alkalis were obtained from natural sources. Soda ash was also produced by burning wood and leaching the ashes with water to obtain a solution that yielded soda ash when the water was boiled off. The name soda ash originates from the barilla plant, which was used to produce soda ash. The scientific name of this plant is Salsola soda, but it goes by the common names of sodawort or glasswort because the soda produced from it was used in making glass. Barilla is a common plant found in saline waters along the Mediterranean Sea in Spain and Italy. Barilla was dried and burned to produce soda ash. The depletion of European forests and international disputes made the availability of alkali salts increasingly uncertain during the latter part of the 18th century. LeBlanc proposed a procedure in 1783, and a plant based on LeBlanc’s method was opened in 1791. Unfortunately, LeBlanc’s association with French Royalty led to the confi scation of the plant at the time of the French Revolution. Furthermore, confl icting claims for LeBlanc’s method were made by several other chemists and he never received the reward.
Uses
Soda ash is used in glass making, in production of sodium chemicals (such as sodium chromates, phosphates, and silicates), in the wood pulp industry, in production of soaps and detergents, in oil refining, in water softening, and in refining of nonferrous metals. In its hydrous crystallized form (Na2C03.10H2O), it is known as sal soda,washing soda,or soda crystals, not to be confused with baking soda,which is sodium hydrogen carbonate or sodium bicarbonate (NaHC03). Its monohydrate form(Na2C03·H20) is the standard compound for scouring solutions.
When in solution, sodium carbonate creates less alkalinity than the hydroxides. A 0.1% solution creates a pH of 11;a fully saturated solution is 35%, which has a pH of 12.5.
The safety requirements for sodium carbonate, because of its lower alkalinity, can be considered less demanding than those for the related bicarbonates.
Production Methods
Sodium carbonate is produced on all continents of the world from its minerals. It is present in large deposits in Africa and the United States as either carbonate or trona, a mixed ore of equal molar amounts of carbonate and bicarbonate. However, about 70% of the world production of sodium carbonate is manufactured by the Solvay (ammonia soda) process, whereby ammonia is added to a solution of sodium chloride. Carbon dioxide is then bubbled through to precipitate the bicarbonate (NaHCO3) that is decomposed by heat-producing sodium carbonate. In the United States. all production is based on the minerals that contain sodium carbonate. Different qualities of sodium carbonate are produced: technical, food, and pharmaceutical grades.
Definition
Sodium carbonate is an organic sodium salt and a carbonate salt. A dibasic acid formed in small amounts in solution when carbon dioxide dissolves in water: CO2 + H2O=H2CO2It forms two series of salts: hydrogencarbonates (HCO3–) and carbonates (CO32-). The pure acid cannot be isolated.
General Description
Sodium carbonate is a water soluble inorganic salt commonly used as a weak base. Its aqueous solution has the ability to uptake carbon dioxide. It can also catalyze the conversion of sewage sludge to liquid fuels.
Safety Profile
Poison by intraperitoneal route. Moderately toxic by inhalation and subcutaneous routes. Mlldly toxic by ingestion. Experimental reproductive effects. A skin and eye irritant. It migrates to food from packagmg materials. Can react violently with Al, P2O5, H2SO4, F2, Li, 2,4,6-trinitrotoluene. When heated to decomposition it emits toxic fumes of Na2O
Purification Methods
It crystallises from water as the decahydrate which is redissolved in water to give a near-saturated solution. By bubbling CO2, NaHCO3 is precipitated. It is filtered off, washed and ignited for 2hours at 280o [MacLaren & Swinehart J Am Chem Soc 73 1822 1951]. Before being used as a volumetric standard, analytical grade material should be dried by heating at 260-270o for 0.5hour and allowed to cool in a desiccator. It has a transition point at 450o, and its solubility in water is 21.58% at 20o (decahydrate in solid phase), 49.25% at 35o (heptahydrate in solid phase) and 44.88% at 75o(monohydrate in solid phase) [D.nges in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 987-988 1963]. After three recrystallisations, technical grade Na2CO3 had Cr, Mg, K, P, Al, W, Sc and Ti at 32, 9.4, 6.6, 3.6, 2.4, 0.6, 0.2 and 0.2 ppm respectively; another technical source had Cr, Mg, Mo, P, Si, Sn and Ti at 2.6, 0.4, 4.2, 13.4, 32, 0.6, 0.8 ppm respectively.
Sodium carbonate Preparation Products And Raw materials
Raw materials
1of5
chevron_rightPreparation Products
1of8
chevron_right| Supplier | Tel | Country | ProdList | Advantage | Inquiry |
|---|---|---|---|---|---|
| ANJI BIOSCIENCES | +91-9000100077 +91-9000100077 | Hyderabad, India | 430 | 58 | Inquiry |
| SUKHA CHEMICAL INDUSTRIES | +91-9638875444 +91-9638875444 | Gujarat, India | 26 | 58 | Inquiry |
| Vardhaman P Golechha | 9704422000 | Telangana, India | 294 | 58 | Inquiry |
| Rishi Chemical Works Pvt Ltd | +91-3322290068 +91-3322290071 | Kolkata, India | 43 | 58 | Inquiry |
| ALLIPO CHEMICALS | +91-8238998187 +91-8238998187 | Gujarat, India | 32 | 58 | Inquiry |
| UNILOSA INTERNATINAL PRIVATE LIMITED | +91-9999069917 +91-9999069917 | New Delhi, India | 153 | 58 | Inquiry |
| JSK Chemicals | +919879767970 | Gujarat, India | 3758 | 58 | Inquiry |
| AVA CHEMICALS PVT LTD | +91-8879390046 +91-8879390046 | Maharashtra, India | 74 | 58 | Inquiry |
| Kronox Lab Sciences Pvt Ltd | +91-9313231074 +91-9313231074 | Gujarat, India | 240 | 58 | Inquiry |
| HRV Global Life Sciences | +91-9820219686 +91-9820219686 | Telangana, India | 379 | 58 | Inquiry |
| Supplier | Advantage |
|---|---|
| ANJI BIOSCIENCES | 58 |
| SUKHA CHEMICAL INDUSTRIES | 58 |
| Vardhaman P Golechha | 58 |
| Rishi Chemical Works Pvt Ltd | 58 |
| ALLIPO CHEMICALS | 58 |
| UNILOSA INTERNATINAL PRIVATE LIMITED | 58 |
| JSK Chemicals | 58 |
| AVA CHEMICALS PVT LTD | 58 |
| Kronox Lab Sciences Pvt Ltd | 58 |
| HRV Global Life Sciences | 58 |
Related articles
- Soda Ash Vs Baking Soda: Similarity and Differences
- Soda Ash(Sodium carbonate), Na2CO3.10H2O, is an industrial chemical with the formula Na2CO3. Baking soda, also known as sodium....
- Mar 7,2024
- Sodium carbonate: Applications and Security
- Sodium carbonate is the disodium carbonate salt with an alkaline base. It is widely used in industry.
- Jan 2,2024
- Uses of Sodium carbonate
- Sodium Carbonate is the disodium salt of carbonic acid with alkalinizing property. When dissolved in water, sodium carbonate f....
- Feb 7,2022
Related Qustion
- Q:What is soda ash used for?
- A:Over half of all Soda Ash production is used in glass manufacturing, but it is used in the soap industry, paper production, p....
- Mar 13,2024





