テレフタル酸 化学特性,用途語,生産方法
外観
白色〜わずかにうすい褐色, 結晶性粉末
性質
テレフタル酸は、分子式C8H6O4、分子量166.13、CAS登録番号100-21-0で表わされます。
1. 物理的性質
テレフタル酸は、白色無臭の結晶性粉末です。比重は1.51、蒸気密度は5.74です。融点は無く、引火点260℃、402℃で昇華、496℃で自然発火する可燃性の個体 (ガス) です。
燃焼性が低いため、繊維や樹脂の製造に適しています。また、高温下でも変質しにくいことも特徴の1つです。
2. 化学的性質
水への溶解度は0.28g/100mLであり、ほとんど溶解しません。エタノールには極めて溶けにくく、水酸化ナトリウム溶液にはやや溶けやすい性質を持ちます。
通常の取扱いにおいては安全ですが、強酸化剤との接触により激しく反応し、燃焼によって一酸化炭素や、二酸化炭素を発生させるため、高温、強酸化剤との接触を避ける必要があります。
溶解性
水, エタノール, エーテルに不溶。DMF, アルカリに可溶。1mol/水酸化ナトリウム溶液にやや溶けやすく、エタノールに極めて溶けにくく、水にほとんど溶けない。
解説
芳香族ジカルボン酸の一つ。テレフタル酸は,1,4-ベンゼンジカルボン酸ともよばれる。フタル酸(1,2-ベンゼンジカルボン酸)およびイソフタル酸(1,3-ベンゼンジカルボン酸)はテレフタル酸の異性体である。実験室的には、p(パラ)-キシレンを過マンガン酸カリウム、三酸化クロムなどにより酸化すると得られる。以前は、フタル酸または安息香酸のカリウム塩を二酸化炭素と加圧・加熱するヘンケル法(ドイツのヘンケル社Henkel & Cieが開発)により工業的に製造されていたが、今日では、コバルトなどの重金属触媒を用いるp-キシレンの空気酸化により製造されている。常圧では300℃付近で昇華する。白色の結晶で、水、エタノール(エチルアルコール)にはほとんど溶けず、ベンゼン、エーテルにも溶けないが、アルカリ水溶液には塩を生成して溶解する。テレフタル酸とグリコールのポリエステル(ポリエチレンテレフタレート、PET)は、日本では「テトロン」の商品名で知られ、合成繊維として多量に生産されているほかに、飲料容器用の合成樹脂(ペットボトル)として広く用いられている。また、1価の脂肪族アルコールとのエステルはプラスチックの可塑剤として用いられている。
用途
合成樹脂原料、塗料原料
用途
ポリエステル系合繊(テトロン)、テトロンフィルム、エンプラ(ポリアリレート)の原料
製造法
1,4-benzenedicarboxylic acid.C8H6O4(166.14).p-ジアルキルベンゼンを酢酸溶媒中,酢酸コバルト-酢酸マンガン触媒と促進剤の臭素化合物を用いて液相空気酸化するか,フタル酸のジカリウム塩を高温で異性化させると得られる.白色の結晶.融点425 ℃(封管中).300 ℃ で昇華する.pK1 3.54,pK2 4.46.普通の有機溶媒や水に不溶,熱濃硫酸,ピリジン,ジメチルホルムアミドに可溶.エチレングリコールと縮重合させて,ポリエステル系の合成繊維または樹脂とする.そのほか,高級アルコールエステルは可塑剤に用いられる.LD50 4390 mg/kg(ラット,経口).[CAS 100-21-0]
森北出版「化学辞典(第2版)
説明
Terephthalic acid is the organic compound with formula C
6H
4(COOH)
2. This colourless solid is a commodity chemical, used principally as a precursor to the polyester PET, used to make clothing and plastic bottles. Several million tones are produced annually. It is one of three isomeric phthalic acids.
化学的特性
Terephthalic acid is a white crystalline solid. Soluble in alkaline solution, slightly soluble in hot ethanol, insoluble in water, ether, glacial acetic acid and chloroform, consequently up until around 1970 most crude terephthalic acid was converted to the dimethyl ester for purification. It sublimates when heated.
来歴
Terephthalic acid came to prominence through the work of Winfield and Dickson in Britain around 1940. Earlier work by Carothers and coworkers in the United States established the feasibility of producing high molecular weight linear polyesters by reacting diacids with diols, but they used aliphatic diacids and diols. These made polyesters which were unsuitable to be spun into fibers. Winfield and Dickson found that symmetrical aromatic diacids yield high-melting, crystalline, and fiberforming materials; poly(ethylene terephthalate) (PET) has since become the largest volume synthetic fiber.
使用
Terephthalic acid (TPA) is a benzenepolycarboxylic acid with potential anti-hemorrhagic properties. It is a high-tonnage chemical, widely used in the production of synthetic materials, notably polyester fibers (poly-(ethylene terephthalate)).
主な応用
1,4-benzenedicarboxylic acid is mainly used for the production of poly (ethylene terephthalate). Also production of plasticizer dioctyl phthalate (DOTP) and polyester plasticized agents. 1,4-benzenedicarboxylic acid and polyhydric alcohols have a condensation reaction withd iethylene glycol, triethylene glycol, glycerol, propylene glycol, butylene glycol, etc. preparation of the polyester plasticizer.
製造方法
The major commercial route to terephthalic acid which is suitable for the
direct preparation of poly(ethylene terephthalate) is from p-xylene:
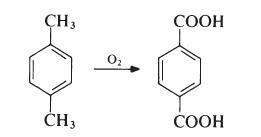
p-Xylene is obtained largely from petroleum sources, being a product of the
fractionation of reformed naphthas. The oxidation is carried
out in the liquid phase. Typically, air is passed into a solution of p-xylene in
acetic acid at about 200?? and 2 MPa (20 atmospheres) in the presence of a
catalyst system containing cobalt and manganese salts and a source of
bromide ions. The terephthalic acid produced contains only small amounts of
impurities (mainly p-carboxybenzaldehyde), which are readily removed. The
acid is dissolved in water at about 2500 e and 5 MPa (50 atmospheres) and
treated with hydrogen (which converts the aldehyde to p-toluic acid). The
solution is then cooled to 100?? and pure terephthalic acid crystallizes.
定義
ChEBI: Terephthalic acid is a benzenedicarboxylic acid carrying carboxy groups at positions 1 and 4. One of three possible isomers of benzenedicarboxylic acid, the others being phthalic and isophthalic acids. It is a conjugate acid of a terephthalate(1-).
主な応用
Virtually the entire world's supply of terephthalic acid and dimethyl terephthalate are consumed as precursors to polyethylene terephthalate (PET). World production in 1970 was around 1.75 million tones. By 2006, global purified terephthalic acid (PTA) demand had exceeded 30 million tonnes.
There is a smaller, but nevertheless significant, demand for terephthalic acid in the production of poly butylene terephthalate and several other engineering polymers.
調製方法
Terephthalic acid is produced by oxidation of p-xylene by oxygen in air:
This reaction proceeds through a p-toluic acid intermediate which is then oxidized to terephthalic acid. In p-toluic acid, deactivation of the methyl by the electron withdrawing carboxylic acid group makes the methyl one tenth as reactive as xylene itself, making the second oxidation significantly more difficult . The commercial process utilizes acetic acid as solvent and a catalyst composed of cobalt and manganese salts, with a bromide promoter.
安全性
眼に対する重篤な損傷性、眼刺激性、皮膚刺激性があり、呼吸器系への刺激の恐れもあります。長期、又は反復暴露による呼吸器系への臓器障害の危険性もあるため、取扱いには注意が必要です。
また、生殖能または胎児への悪影響の疑いがあることから、誤って吸引、経口摂取した際は直ちに医師の診断、手当を受けることが重要です。
一般的な説明
White powder.
空気と水の反応
Insoluble in water.
反応プロフィール
Terephthalic acid is a carboxylic acid. Terephthalic acid donates hydrogen ions if a base is present to accept them. This "neutralization" generates substantial amounts of heat and produces water plus a salt. Insoluble in water but even "insoluble" carboxylic acids may absorb enough water from the air and dissolve sufficiently in Terephthalic acid to corrode or dissolve iron, steel, and aluminum parts and containers. May react with cyanide salts to generate gaseous hydrogen cyanide. Will react with solutions of cyanides to cause the release of gaseous hydrogen cyanide. Flammable and/or toxic gases and heat are generated by reaction with diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides. React with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), to generate flammable and/or toxic gases and heat. Reaction with carbonates and bicarbonates generates a harmless gas (carbon dioxide) but still heat. Can be oxidized by strong oxidizing agents and reduced by strong reducing agents. These reactions generate heat. May initiate polymerization reactions; may catalyze (increase the rate of) chemical reactions.
火災危険
Flash point data for Terephthalic acid are not available. Terephthalic acid is probably combustible.
使用用途
テレフタル酸は、化学繊維の中で最も多く使用されているポリエステル繊維、各種工業製品で基盤フィルムと使用されるPET () 樹脂、および飲料容器として幅広く用いられているPETボトルの原料に利用されています。
化学品中間物、有機化学製品 (合成繊維、合成樹脂) 、ポリエステル系合繊 (テトロン) 、テトロンフィルム (ルミラー、ダイテフォイル) 、ボトルエンプラ (ポリアリレート) の原料にも有用です。その他、医療用のステントや人工心臓弁などの機械部品、染料、顔料、農薬の原料などにも利用されます。
取扱方法
適切な呼吸器保護具、保護手袋、保護眼鏡 (普通眼鏡型、側板付き普通眼鏡型、ゴーグル型) 、必要に応じて保護衣、保護面、耐薬品性長靴、前掛け (静電気防止対策用) などを着用して作業を行います。
取り扱う作業場には、洗眼器と安全シャワーを設置し、密閉もしくは局所排気装置などの蒸気や粉末が暴露しないよう対策を行う必要があります。
使用時は飲食、喫煙をせず、取扱い後はよく手を洗い皮膚や眼への付着を避けます。
製造??輸出量
テレフタル酸の国内供給量は、1999年以降減少傾向にあり、2003年では製造量1,443,644トンでした。約6割はフィルム、ボトル等の原料に利用され、約4割が繊維に加工されています。
届出排出量及び移動量並びに届出外排出量の集計結果 (平成15年度) によると、1年間で、全国合計で、大気に24kg、公共用水域へ133トン排出され、廃棄物として1,699トン、下水道へ37トン移動していることが報告されています。
排出量のうちほとんどが、繊維工業からの公共用水域への排出です。また全体的に環境への排出量より、廃棄物としての移動量の方が多いです。
環境への影響
テレフタル酸は、加水分解を受けやすい化学結合はないことから、加水分解されません。好気的生分解試験では、生物化学的酸素消費量 (BOD) 測定では74.7%、高速液体クロマトグラフ (HPLC) では100%分解することから、良分解性を持つことが報告されています。
また、嫌気的生分解性試験では、55日後に50%分解することが報告されており、環境中に排出された場合でも容易に生分解され除去されることが推定されています。
参考文献
化学性质
色の针状結晶。酸性を持ち、アミン類と酰胺類と反応する。
安全性プロファイル
Moderately toxic by intravenous and intraperitoneal routes. Mildly toxic by ingestion. An eye irritant, Can explode during preparation. When heated to decomposition it emits acrid smoke and irritating fumes.
職業ばく露
TPA is used primarily in the production of polyethylene terephthalate polymer for the fabrication of polyester fibers and films. A high-volume production chemical in the United States.
合成方法
石油系の中間生成物、例えば對二甲苯を酸化する方法で合成される。
純化方法
Purify the acid via the sodium salt which, after crystallisation from water, is re-converted to the acid by acidification with mineral acid. Filter off the solid, wash it with H2O and dry it in a vacuum. The S-benzylisothiuronium salt has m 204o (from aqueous EtOH). [Beilstein 9 IV 3301.]
不和合性
Combustible; dust may form an explosive mixture with air. Compounds of the carboxyl group react with all bases, both inorganic and organic (i.e., amines) releasing substantial heat, water and a salt that may be harmful. Incompatible with arsenic compounds (releases hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides (releasing heat, toxic and possibly flammable gases), thiosulfates and dithionites (releasing hydrogen sulfate and oxides of sulfur). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
廃棄物の処理
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
テレフタル酸 上流と下流の製品情報
原材料
準備製品