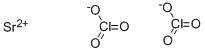스트론튬 염산
|
|
스트론튬 염산 속성
- 녹는점
- 120° with decomposition and evolution of O2
- 밀도
- 3.15
- 용해도
- 에탄올에 약간 용해됨
- 색상
- colorless crystals, crystalline
- 수용성
- g/100g 용액 H2O: 61.40(0°C), 63.78(25°C), 67.08(95°C); 고체상, Sr(ClO3)2 ·3H2O (0°C), Sr(ClO3)2 (25°C, 95°C) [KRU93]; 알코올에 약간 용해됨 [MER06]
안전
| 유엔번호(UN No.) | 1506 | ||
|---|---|---|---|
| 위험 등급 | 5.1 | ||
| 포장분류 | II | ||
| 유해 물질 데이터 | 7791-10-8(Hazardous Substances Data) | ||
| 기존화학 물질 | KE-32214 | ||
| 유해화학물질 필터링 | 97-1-198 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 염소산 염류 및 이를 1% 이상 함유한 혼합물. 다만, 폭발약은 제외 |
스트론튬 염산 C화학적 특성, 용도, 생산
개요
Strontium chlorate, Sr(ClO3)2, has a molecular weight of 254.52 g/mol. It is a white solid with a melting point of 120°C and a density of 3.152 g/cm3. It is soluble in water (174 g/100 ml at 20°C). Its CAS number is 7791-10-8. Sr(ClO3)2 is shock sensitive, and is highly flammable if allowed to sit in air. These white, water-soluble crystals decompose at 120°C. It is used in pyrotechnics and tracer bullets. There are two coexisting crystal hydrates that undergo interconversion, the dihydrate and the hexahydrate. These compounds are not as shock sensitive as the anhydrate.화학적 성질
White, crystalline powder. Soluble in water; slightly soluble in alcohol.물리적 성질
Strontium chlorate is a strong oxidizing agent. It forms a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive.Contact with concentrated sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. This compound liberates explosive chlorine dioxide gas in the presence of strong acid; heating with a dibasic organic acid in the presence of moisture liberates chlorine dioxide and carbon dioxide. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive.
용도
In pyrotechnics to produce red fire.제조 방법
Strontium chlorate can be produced by the Liebig method which consists of passing chlorine gas through a solid such as Sr(OH)2:6Sr(OH)2+ 6Cl2→5SrCl2+ Sr(CIO3)2+ H2O.
However, separating the two salts remains problematic since both are soluble in water. Strontium chloride solubility is 42.9 g/100 ml of water at 20°C while that of strontium chlorate is 174.9 g/100 ml of water at 20°C.
일반 설명
A moist solid or semi-solid slurry of white crystals. May explode under exposure to heat or fire. Used in pyrotechnics.공기와 물의 반응
Water soluble.반응 프로필
STRONTIUM CHLORATE is a strong oxidizing agent. Forms a flammable mixture with combustible materials (can be ignited by friction). Such mixtures may be explosive Contact with concentrated sulfuric acid can cause fires or explosions. When mixed with ammonium salts, spontaneous decomposition and ignition may result. Liberates explosive chlorine dioxide gas in the presence of strong acid; heating with a dibasic organic acid in the presence of moisture liberates chlorine dioxide and carbon dioxide [Bretherick 1979 p. 100]. Mixtures with ammonium salts, powdered metals, silicon, sulfur, or sulfides are readily ignited and potentially explosive [Bretherick 1979 p. 806].위험도
Dangerous explosion risk in contact with organic materials; highly sensitive to shock, heat, and friction; strong oxidizing agent.건강위험
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Fire may produce irritating and/or toxic gases. Toxic fumes or dust may accumulate in confined areas (basement, tanks, hopper/tank cars, etc.). Runoff from fire control or dilution water may cause pollution.화재위험
May explode from friction, heat or contamination. These substances will accelerate burning when involved in a fire. May ignite combustibles (wood, paper, oil, clothing, etc.). Some will react explosively with hydrocarbons (fuels). Containers may explode when heated. Runoff may create fire or explosion hazard.Safety Profile
A powerful oxidizer. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORATES.스트론튬 염산 준비 용품 및 원자재
원자재
준비 용품
스트론튬 염산 공급 업체
글로벌( 15)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28180 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-61398051 +8613650506873 |
sales@chemdad.com | China | 39916 | 58 |
| Hefei TNJ Chemical Industry Co.,Ltd. | 0551-65418671 |
sales@tnjchem.com | China | 34572 | 58 |
| Baoji Guokang Healthchem co.,ltd | +8615604608665 15604608665 |
dominicguo@gk-bio.com | CHINA | 9427 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Chengdu HuaXia Chemical Reagent Co. Ltd | 400-1166-196 13458535857 |
cdhxsj@163.com | China | 13358 | 58 |
| Shandong Xiya Chemical Co., Ltd. | 4009903999 13395398332 |
sales@xiyashiji.com | China | 20810 | 60 |
| Shaanxi DIDU pharmaceutical and Chemical Co., Ltd | 17691182729 18161915376 |
1046@dideu.com | China | 10008 | 58 |
스트론튬 염산 관련 검색:
차아염소산 칼슘 염소산 마그네슘 염소산 칼슘 과염소산스트론듐 스트론튬 염산
STRONTIUM BROMIDE HEXAHYDRATE
STRONTIUM ALUMINATE
STRONTIUM BORIDE
Calcium bromide
Calcium chlorite
magnesium hypochlorite
Barium hypochlorite
BariumChlorite
beryllium perchlorate
MAGNESIUM CHLORITE
BERYLLIUM CHLORATE
Dihypochlorous acid strontium salt
Bischlorous acid strontium salt