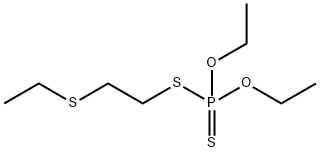
에틸티오메톤
|
|
에틸티오메톤 속성
- 녹는점
- 110-112℃
- 끓는 점
- bp0.01 108°; bp1.5 132-133°
- 밀도
- 1.1445 g/cm3 (20 ºC)
- 증기압
- 7.2 x 10-3 Pa (20 °C)
- 굴절률
- 1.5501 (589.3 nm 20℃)
- 인화점
- 133 °C
- 저장 조건
- 0-6°C
- 수용성
- 25mg l-1(20°C)
- 물리적 상태
- 액체
- Merck
- 13,3400
- BRN
- 1709167
- CAS 데이터베이스
- 298-04-4(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 27/28-50/53 | ||
| 안전지침서 | 28-36/37-45-60-61 | ||
| 유엔번호(UN No.) | 3018 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TD9275000 | ||
| 위험 등급 | 6.1(a) | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 298-04-4(Hazardous Substances Data) | ||
| 독성 | LD50 in female, male rats (mg/kg): 2.3, 6.8 orally; 6, 15 dermally (Gaines) | ||
| 기존화학 물질 | KE-10474 | ||
| 유해화학물질 필터링 | 97-1-37;06-4-5 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 디술포톤 및 이를 1% 이상 함유한 혼합물 |
| 그림문자(GHS): |
 
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 신호 어: | Danger | ||||||||||||||
| 유해·위험 문구: |
|
||||||||||||||
| 예방조치문구: |
|
에틸티오메톤 C화학적 특성, 용도, 생산
화학적 성질
Disulfoton is a dark yellowish oil with an aromatic, sulfurous odor. It is soluble in most organic solvents and fatty oils. Disulfoton is a selective, systemic insecticide and acaricide. It is used for seed coating and for soil application to protect from insect attacks, for the control of sucking insects, aphids, leaf hoppers, thrips, beet-fl ies, spider mites, and coffeeleaf miners. Disulfoton has been used extensively in pest control on a variety of crops, such as cotton, tobacco, sugar beets, corn, peanuts, wheat, ornamentals, cereal grains, and potatoes. It is grouped by the US EPA under RUP. Human exposures to disulfoton occur through breathing contaminated air, drinking contaminated water, eating contaminated food, and working in industries that manufacture and formulate the pesticide.용도
DISULFOTON is used as systemic insecticide and acaracide.
정의
ChEBI: An organic thiophosphate that is the diethyl ester of S-[2-(ethylsulfanyl)ethyl] dihydrogen phosphorodithioate.일반 설명
Disulphoton is a dark yellowish oil with an aromatic, sulphurous odour. It is soluble in most organic solvents and fatty oils. Disulphoton is a selective, systemic insecticide and acaricide. It is used for seed coating and for soil application to protect from insect attacks and for the control of sucking insects, aphids, leafhoppers, thrips, beetflies, spider mites, and coffee-leaf miners. Disulphoton has been extensively used in pest control on a variety of crops such as cotton, tobacco, sugar beets, corn, peanuts, wheat, ornamentals, cereal grains, and potatoes. It is grouped by the U.S. EPA under RUP. Human exposures to disulphoton occur through breathing contaminated air, drinking contaminated water, eating contaminated food, and working in industries that manufacture and formulate the pesticide.반응 프로필
Organothiophosphates, such as DISULFOTON, are susceptible to formation of highly toxic and flammable phosphine gas in the presence of strong reducing agents such as hydrides. Partial oxidation by oxidizing agents may result in the release of toxic phosphorus oxides.건강위험
Disulfoton is highly toxic to animals and humans by all routes of exposures, namely, by dermal absorption, through ingestion, and inhalation by the respiratory route. The symptoms of poisoning include blurred vision, fatigue, headache, dizziness, sweating, tearing, and salivation. It inhibits cholinesterase and affects the nervous system function. It does not cause delayed neurotoxicity. Prolonged period of exposures to high concentrations of disulfoton cause harmful effects to the nervous system with symptoms such as narrowing of the pupils, vomiting, diarrhea, drooling, diffi culty in breathing, lung edema, tremors, convulsions, coma, and death. Disulfoton causes no mutagenic or teratogenic effects in laboratory animals. There are no reports indicating that disulfoton causes cancer in animals or humans. The DHHS, the IARC, and the US EPA have not classifi ed disulfoton as to its ability to cause cancer.화재위험
Combustible material: may burn but does not ignite readily. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.농업용
Insecticide, Acaricide: All products formulated at greater than 2% disulfoton are classified as Restricted Use Pesticides (RUP). Disulfoton is a selective, systemic organophosphate insecticide and acaricide that is especially effective against sucking insects. It is used to control aphids, leafhoppers, thrips, beet flies, spider mites, and coffee leaf miners. Not approved for use in EU countries. There are 21 global suppliers.상품명
BAY 19639®; BAYER 19639®; DIMAZ®; DISULFATON®; DI-SYSTON®[C]; DISYSTON®[C]; DISYSTOX®; DITHIODEMETON®; DITHIOSYSTOX®; EKATIN TD®; FRUMIN-AL®; FRUMIN G®; GLEBOFOS®; M-74®; S 276®; SOLVIREX®; THIODEMETON®; THIODEMETRON®Safety Profile
Poison by ingestion, inhalation, skin contact, intraperitoneal, and intravenous routes. Human mutation data reported. When heated to decomposition it emits veq toxic SOx and POx. See also various demeton entries and ESTERS.환경귀착
Soil/Plant. Disulfoton was metabolized in soil and plants to the corresponding sulfoxide and sulfone via oxidation of the thioether sulfur atoms (Metcalf et al., 1957; Getzin and Shanks, 1970; Takase et al., 1972; Clapp et al., 1976; Worthing and Hance, 1991), the corresponding phosphorothioate analogs and then to derivatives of O,O-diethyl hydrogen phosphate and 2-ethylthioethyl mercaptan (Worthing and Hance, 1991). Disulfoton is rapidly oxidized in soil to its sulfoxide and sulfone with disulfoton oxon sulfoxide and disulfoton oxon sulfone appearing in small amounts (Szeto et al., 1983). In a Portneuf silt loam soil, the persistence of the sulfoxide and sulfone was 32 and >64 days, respectively (Clapp et al., 1976).Disulfoton was translocated from a sandy loam soil into asparagus tips. Disulfoton sulfoxide, disulfoton sulfone, disulfoton oxon sulfoxide and disulfoton oxon sulfone were recovered as metabolites (Szeto and Brown, 1982; Szeto et al., 1983). Disulfoton s
Groundwater. According to the U.S. EPA (1986) disulfoton has a high potential to leach to groundwater.
Photolytic. Disulfoton was rapidly oxidized to disulfoton sulfoxide and trace amounts (<5% yield) of disulfoton sulfone when sorbed on soil and exposed to sunlight (half-life 1–4 days) (Gohre and Miller, 1986). The photosensitized oxidation was probably due to the presence of singlet oxygen (Gohre and Miller, 1986; Zepp et al., 1981). The degradation rate was higher in soils containing the lowest organic carbon (Gohre and Miller, 1986). Chemical/Physical. Emits toxic fumes of phosphorus and sulfur oxides when heated to decomposition (Sax and Lewis, 1987; Lewis, 1990).
When fertilizers containing superphosphate and ammonium nitrate were impregnated with disulfoton, the latter chemically degraded to form disulfoton sulfone and disulfoton sulfoxide (Ibrahim et al., 1969).
The reported half-lives for abiotic hydrolysis
신진 대사 경로
Disulfoton is metabolised by an analogous route to phorate. The principal route of disulfoton metabolism in all media is activation via oxidation of the thioether group to the sulfoxide (rapid) and sulfone (slower). Thioether oxidation occurs preferentially to oxidative desulfuration of the P=S group to the oxon, which is usually only present in trace amounts and there is good evidence that the sulfoxide and sulfone oxons arise via disulfoton sulfoxide and sulfone rather than disulfoton oxon. The more polar thiooxidised metabolites are translocated in plants and are responsible for the compound's systemic action.신진 대사
The metabolic routes of disulfoton are essentially the same in plants, insects, and mammals, involving the oxidation of the sulfide group into the sulfoxide and then sulfone, oxidative desulfuration to the corresponding oxons, and hydrolysis to diethyl phosphorothioate. In mammals, orally administered disulfoton is rapidly metabolized and excreted in the urine. Disulfoton is rapidly degraded in soil; DT50 (20 ?C) was 1.3–2 d.에틸티오메톤 준비 용품 및 원자재
원자재
준비 용품
에틸티오메톤 관련 검색:
3,4-자일렌올 데메톤-S 에틸티오메톤 옥시디술포톤 티오메톤 디알킬디티오인산
Diethylphosphorodithioate
DISULFOTON-SULFONE,DISULFOTON SULFONE, 100MG,NEAT,DISULFOTON-SULFONE PESTANAL, 100 MG
O,O,S-TRIMETHYLDITHIOPHOSPHATE
DISULFOTON SULFONE
DISULFOTON D10 (DI-ETHYL D5)
DISULFOTON SULFOXIDE
DISULFOTON-OXON-SULFOXIDE
DISULFOTON, 1X1ML, CH2CL2, 2000 UG/ML
DISULFOTON SOLUTION 100UG/ML IN METHANOL 1ML
DISULFOTON SOLUTION 100UG/ML IN METHANOL 5X1ML
DISULFOTON SOLUTION 100UG/ML IN METHANOL 5ML
O,O-Diethyl S-methyl dithiophosphate