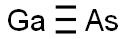갈륨 아르세니드
|
|
갈륨 아르세니드 속성
- 녹는점
- 1238°C
- 밀도
- 5.31 g/mL at 25 °C (lit.)
- 증기압
- 0Pa at 20℃
- 굴절률
- 3.57
- 물리적 상태
- 플레이크 크리스탈
- 색상
- 짙은 회색
- Specific Gravity
- 5.31
- 비저항
- ≥1E7 Ω-cm
- 수용성
- 염산에 용해됩니다. 물, 에탄올, 메탄올 및 아세톤에 용해되지 않습니다.
- Crystal Structure
- Cubic, Sphalerite Structure - Space Group F(-4)3m
- Merck
- 14,4347
- CAS 데이터베이스
- 1303-00-0(CAS DataBase Reference)
- IARC
- (Vol. 86, 100C) 2012
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 23/25-50/53-48/23-45 | ||
| 안전지침서 | 20/21-28-45-60-61-53 | ||
| 유엔번호(UN No.) | UN 1557 6.1/PG 2 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | LW8800000 | ||
| F 고인화성물질 | 10 | ||
| TSCA | Yes | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | II | ||
| HS 번호 | 2853909090 | ||
| 유해 물질 데이터 | 1303-00-0(Hazardous Substances Data) | ||
| 독성 | mouse,LD50,intraperitoneal,4700mg/kg (4700mg/kg),BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY)PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE)BEHAVIORAL: FOOD INTAKE (ANIMAL),Gigiena i Sanitariya. For English translation, see HYSAAV. Vol. 45(10), Pg. 13, 1980. | ||
| 기존화학 물질 | KE-17426 | ||
| 유해화학물질 필터링 | 97-1-119 | ||
| 중점관리물질 필터링 | 별표1-97 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 비소 또는 그 화합물과 비소화합물을 0.1% 이상 함유한 혼합물 |
갈륨 아르세니드 C화학적 특성, 용도, 생산
화학적 성질
Gallium arsenide contains 48.2% gallium and 51.8% arsenic and is considered an intermetallic compound. It occurs as cubic crystals with a dark gray metallic sheen. Gallium arsenide is electroluminescent in infrared light. Gallium arsenide readily reacts with oxygen in air, forming a mixture of oxides of gallium and arsenic on the crystal surface. Gallium arsenide presents the following properties: hardness, 4.5; thermal expansion coefficient, 5.9×106; thermal conductivity, 0.52Wunits; specific heat, 0.086 cal/g/ ℃; intrinsic electron concentration, 107; energy gap at room temperature, 1.38 eV; electron mobility, 8800 cm2/V/s; effective mass for electrons, 0.06m0; lattice constant,5.6–54 AO; dielectric constant, 11.1; intrinsic resistivity at 300K=3.7×108Ωcm; electron lattice mobility at 300K= 10,000 cm2/V/s; intrinsic charge density at 300K=1.4 106 cm-3; electron diffusion constant at 300K=310 cm2/s; and hole diffusion constant=11.5 cm2/s.
Garlic odor when moistened. Finely divided gallium arsenide can react vigorously with steam, energetic acids, and oxidizers to evolve arsine gas, and can release arsenic fumes when heated to decomposition. The molten form attacks quartz.
물리적 성질
Gray cubic crystal; density 5.316 g/cm3; melts at 1,227°C; hardness 4.5 Mohs; lattice constant 5.653?; dielectric constant 11.1; resistivity (intrinsic) at 27°C, 3.7x108 ohm-cm.용도
In semiconductor applications (transistors, solar cells, lasers).Gallium arsenide is among the most widely used intermetallic semiconductor components (Harrison, 1986; McIntyr and Sherin, 1989). Gallium arsenide is also incorporated into light-emitting diodes and photovoltaic cells, while gallium alloys are used for dental amalgam as a low toxicity replacement for mercury.제조 방법
Gallium arsenide is prepared by passing a mixture of arsenic vapor and hydrogen over gallium(III) oxide heated at 600°C: Ga2O3 + 2As + 3H2 2GaAs + 3H2OThe molten material attacks quartz. Therefore, quartz boats coated with carbon by pyrolytic decomposition of methane should be used in refining the compound to obtain high purity material. Gallium arsenide is produced in polycrystalline form as high purity, single crystals for electronic applications. It is produced as ingots or alloys, combined with indium arsenide or gallium phosphide, for semiconductor applications.일반 설명
Dark gray crystals with a metallic greenish-blue sheen or gray powder. Melting point 85.6°F (29.78°C).공기와 물의 반응
Stable in dry air. Tarnishes in moist air. Insoluble in water.반응 프로필
GALLIUM ARSENIDE can react with steam, acids and acid fumes. Reacts with bases with evolution of hydrogen. Attacked by cold concentrated hydrochloric acid. Readily attacked by the halogens. The molten form attacks quartz.위험도
Toxic metal. Questionable carcinogen화재위험
Flash point data for GALLIUM ARSENIDE are not available; however, GALLIUM ARSENIDE is probably combustible.Safety Profile
Confirmed carcinogen. Mddly toxic by intraperitoneal route. Most arsenic compounds are poisons. Can react with steam, acids, and acid fumes to evolve the deadly poisonous arsine. Molten gallium arsenide attacks quartz. When heated to decomposition it emits very toxic fumes of As. See also ARSENIC COMPOUNDS and GALLIUM COMPOUNDS.Carcinogenicity
Carcinogenesis.Gallium is antineoplastic in several human and murine cancer cell lines and in some in vivo cancers. It has been used experimentally in patients in the treatment of lymphatic malignancies (including multiple myelomas) and for urothelial malignancies. However, the dissociation of gallium arsenide into gallium and arsenic is a factor to take into account. A considerable number of studies have evaluated the carcinogenic potential of arsenic and various arsenic compounds.
Structure and conformation
The space lattice of gallium arsenide (GaAs) belongs to the cubic system Td2, and its zincblende-type structure has a lattice constant of a=0.5654 nm and a distance to its nearest neighbor of 0.244 nm.갈륨 아르세니드 준비 용품 및 원자재
원자재
준비 용품
갈륨 아르세니드 공급 업체
글로벌( 82)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21667 | 55 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
sales@coreychem.com | China | 29888 | 58 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| Chongqing Chemdad Co., Ltd | +86-023-6139-8061 +86-86-13650506873 |
sales@chemdad.com | China | 39916 | 58 |
| changzhou huayang technology co., ltd | +8615250961469 |
2571773637@qq.com | China | 9827 | 58 |
| Shaanxi Didu New Materials Co. Ltd | +86-89586680 +86-13289823923 |
1026@dideu.com | China | 8672 | 58 |
| GIHI CHEMICALS CO.,LIMITED | +8618058761490 |
info@gihichemicals.com | China | 50003 | 58 |
| Aladdin Scientific | +1-+1(833)-552-7181 |
sales@aladdinsci.com | United States | 57511 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 021-61259108 18621169109 |
market03@meryer.com | China | 40240 | 62 |