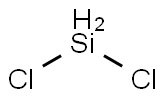
디클로로실란
|
|
디클로로실란 속성
- 녹는점
- −122 °C(lit.)
- 끓는 점
- 8.3 °C(lit.)
- 밀도
- 1,22 g/cm3
- 증기 밀도
- 3.5 (vs air)
- 증기압
- 1254 mm Hg ( 20 °C)
- 인화점
- -37°C
- 용해도
- reacts with H2O
- 물리적 상태
- 무색 가스
- 색상
- colorless gas; flammable
- Specific Gravity
- 0.76
- 폭발한계
- 99%
- 수용성
- 물에서 분해되다
- Hydrolytic Sensitivity
- 9: reacts extremely rapidly with atmospheric moisture - may be pyrophoric - glove box or sealed system required
- 안정성
- 안정적인. 가연성이 매우 높습니다. 폭발 한계가 매우 넓다는 점에 유의하십시오. 물, 알코올, 강산화제, 염기와 격렬하게 반응함.
- CAS 데이터베이스
- 4109-96-0(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | F+,T | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 12-14-23-34 | ||
| 안전지침서 | 26-36/37/39-45 | ||
| 유엔번호(UN No.) | UN 2189 2.3 | ||
| WGK 독일 | 1 | ||
| RTECS 번호 | VV3050000 | ||
| F 고인화성물질 | 4.5 | ||
| TSCA | Yes | ||
| DOT ClassificationII | 2.3 Hazard Zone B (Gas poisonous by inhalation) | ||
| 위험 등급 | 2.3 | ||
| 독성 | mouse,LC50,inhalation,144ppm/4H (144ppm),Archives of Toxicology. Vol. 70, Pg. 218, 1996. | ||
| 기존화학 물질 | KE-10209 | ||
| 사고대비 물질 필터링 | 89 |
디클로로실란 C화학적 특성, 용도, 생산
개요
Dichlorosilane is a highly flammable, corrosive, and toxic gas at room temperature and atmospheric pressure. It causes severe bums on contact with eyes, skin, and mucous membranes. With water or moisture, it hydrolyzes rapidly to yield silica and silicon oxyhydride along with hydrochloric acid. It is shipped as a liquefied gas in low pressure cylinders at its vapor pressure of 9.1 psig (62.7 kPa) at 70°F (21.1℃. It can form flammable mixtures with air and oxidizing agents.화학적 성질
colourless gas용도
Dichlorosilane is primarily used in the electronics industry for such applications as growth of epitaxial or polycrystalline silicon and chemical vapor deposition of silicon dioxide and silicon nitride.일반 설명
Dichlorosilane is a flammable and poisonous gas, with a strong repulsive odor. Dichlorosilane is easily ignited in air, reacts with oxidizing agents, is very toxic by inhalation, and is a strong irritant to skin, eyes and mucous membranes. Under prolonged exposure to fire or intense heat the container may rupture violently or rocket.공기와 물의 반응
Highly flammable. Based on the properties of similar materials, there is the possibility that the reaction of Dichlorosilane with water may be vigorous or violent. Products of the reaction include hydrogen chloride. The reaction generates heat and this heat may be sufficient to ignite the product. The chlorosilicon hydrides(ClxSiHy) are spontaneously flammable in air, NFPA 1991.반응 프로필
Chlorosilanes, such as Dichlorosilane, are compounds in which silicon is bonded to from one to four chlorine atoms with other bonds to hydrogen and/or alkyl groups. Chlorosilanes react with water, moist air, or steam to produce heat and toxic, corrosive fumes of hydrogen chloride. They may also produce flammable gaseous H2. They can serve as chlorination agents. Chlorosilanes react vigorously with both organic and inorganic acids and with bases to generate toxic or flammable gases.위험도
Dichlorosilane is toxic by inhalation and skin absorption. Hydrogen chloride causes severe eye and skin burns and is irritating to the skin, eyes, and respiratory system. The four-digit UN identification number is 2189. The NFPA 704 designation is health 4, flammability 4, and reactivity 2. The white area at the bottom of the diamond contains a W with a slash through it, indicating water reactivity.건강위험
TOXIC; may be fatal if inhaled or absorbed through skin. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.화재위험
Flammable; may be ignited by heat, sparks or flames. May form explosive mixtures with air. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Some of these materials may react violently with water. Cylinders exposed to fire may vent and release toxic and flammable gas through pressure relief devices. Containers may explode when heated. Ruptured cylinders may rocket. Runoff may create fire or explosion hazard.Materials Uses
Dichlorosilane, in the complete absence of water, can be safely stored in mild steel equipment. In the presence of even small traces of water, dichlorosilane becomes extremely corrosive since the Si-CI bonds react rapidly with water, generating hydrogen chloride.br/> Because of reactivity with water, dichlorosilane should always be handled in dry equipment with a dry inert gas such as nitrogen. For transfer service, dry inert gas is preferred to pumping. Some examples of other common compatible materials used include: Viton, Teflon, Kel-F, nickel, Monel, and some types of stainless steel.Safety Profile
Moderately toxic by inhalation. Ignites spontaneously in air. Confined mixtures with air are spontaneously explosive. When heated to decomposition it emits toxic fumes of Cl-. See also CHLOROSILANES.저장
Since dichlorosilane is a highly flammable, corrosive, and toxic liquefied gas, appropriate precautions must be taken in its storage and handling. During the handling of chlorosilanes, the use of such protective equipment as goggles, neoprene or natural rubber gloves, and protective clothing is essential. SCBAs, as well as both safety showers and eyewash fountains, should be available for emergency use.Cylinders should be assigned a definite area for storage. The area should be dry, cool, well ventilated, fire resistant, and away from ignition sources. Keep cylinders protected from excessive temperature rise by storing them away from radiators or other heat sources. Storage conditions should comply with local and state regulations.
Cylinders may be stored in the open, but must be protected against extremes of weather and from the dampness of the ground to prevent rusting. During the summer, cylinders stored in the open should be shaded against the continuous direct rays of the sun in those localities where extreme temperatures prevail.
폐기물 처리
Dichlorosilane should not be discharged directly into surface waters or sewer systems since an acidic waste product is formed. The disposal can be accomplished by controlled introduction of the product into water. The exothermic reactions of dichlorosilane with water (hydrolysis) result in the formation of hydrochloric acid and an insoluble silicon containing solid or fluid. In order to prevent air pollution, the quantity of water must be sufticient to dissolve all of the hydrogen chloride that will be formed. The ratio of water to dichlorosilane should be at least 10 to 1. The corrosive and exothermic nature of the reaction should be t;onsidered in selecting materials of construction for the equipment used in this procedure.The hydrochloric acid formed should then be neutralized with an alkali agent such as aqueous ammonia, sodium hydroxide, lime slurry, etc., and should be added as an aqueous solution with agitation to the acidic medium. Consideration must be given to the additional heat that will be produced by the neutralization. Silicon-containing solids should be washed to remove residual acid. Discard any product, residue, disposable container, or liner in an environmentally acceptable manner. Disposal of dichlorosilane by neutralizing, scrubbing, incineration, or by other means, may be subject to permitting by federal, state or provincial regulations. Persons involved with disposal of dichiorosilane should check with the environmental authorities having jurisdiction to determine the applicability of permitting regulations to disposal activities.
디클로로실란 준비 용품 및 원자재
원자재
준비 용품
디클로로실란 공급 업체
글로벌( 61)공급 업체
| 공급자 | 전화 | 이메일 | 국가 | 제품 수 | 이점 |
|---|---|---|---|---|---|
| ATK CHEMICAL COMPANY LIMITED | +undefined-21-51877795 |
ivan@atkchemical.com | China | 32480 | 60 |
| Hubei xin bonus chemical co. LTD | 86-13657291602 |
linda@hubeijusheng.com | CHINA | 22968 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49390 | 58 |
| WinWin Chemical CO., Limited | +86-0086-577-64498589 +86-15355981851 |
sales@win-winchemical.com | China | 13854 | 58 |
| Henan Alfa Chemical Co., Ltd | +8618339805032 |
alfa4@alfachem.cn | China | 12755 | 58 |
| VladaChem GmbH | +49-7246-3082843 |
info@vladachem.de | Germany | 1860 | 58 |
| Mainchem Co., Ltd. | +86-0592-6210733 |
sale@mainchem.com | China | 32360 | 55 |
| Chemwill Asia Co.,Ltd. | 86-21-51086038 |
chemwill_asia@126.com | CHINA | 23931 | 58 |
| Meryer (Shanghai) Chemical Technology Co., Ltd. | 4006608290; 18621169109 |
market03@meryer.com | China | 40241 | 62 |
| Energy Chemical | 021-021-58432009 400-005-6266 |
sales8178@energy-chemical.com | China | 44751 | 61 |