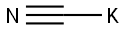사이안화 칼륨
|
|
사이안화 칼륨 속성
- 녹는점
- 634 °C (lit.)
- 끓는 점
- 1625 °C
- 밀도
- 1.00 g/mL at 20 °C
- 증기압
- 1.8hPa at 634.5℃
- 인화점
- 1625°C
- 저장 조건
- Poison room
- 용해도
- H2O: 1 M at 20 °C, 투명, 무색
- 산도 계수 (pKa)
- 9.36[at 20 ℃]
- 물리적 상태
- 고체
- 물리적 상태
- 단단한 모양
- 색상
- 하얀색
- Specific Gravity
- 1.52
- 수소이온지수(pH)
- 11-12 (20g/l, H2O, 20°C)
- 수용성
- 물에 잘 녹습니다. 메탄올, 글리세롤 및 포름아미드에 용해됩니다. 에탄올에 약간 용해됩니다.
- 감도
- Hygroscopic
- Merck
- 14,7626
- BRN
- 4652394
- 노출 한도
- TLV-TWA (measured as CN) skin 5 mg CN/m3 (ACGIH and OSHA); 5 mg CN/m3/ 10 min ceiling (NIOSH).
- 안정성
- 안정적인. 산, 요오드, 과산화물, 과망간산염, 알칼로이드, 염소 수화물, 금속염을 포함한 다양한 물질과 호환되지 않습니다. 빛과 습기에 민감합니다. 산과 접촉하면 독성이 매우 강한 HCN 가스가 생성됩니다.
- LogP
- -0.252 at 20℃
- CAS 데이터베이스
- 151-50-8(CAS DataBase Reference)
안전
- 위험 및 안전 성명
- 위험 및 사전주의 사항 (GHS)
| 위험품 표기 | T+,N | ||
|---|---|---|---|
| 위험 카페고리 넘버 | 26/27/28-32-50/53 | ||
| 안전지침서 | 7-28-29-45-60-61 | ||
| 유엔번호(UN No.) | UN 1680 6.1/PG 1 | ||
| WGK 독일 | 3 | ||
| RTECS 번호 | TS8750000 | ||
| F 고인화성물질 | 3-8-10-23 | ||
| TSCA | Yes | ||
| HS 번호 | 2837 19 00 | ||
| 위험 등급 | 6.1 | ||
| 포장분류 | I | ||
| 유해 물질 데이터 | 151-50-8(Hazardous Substances Data) | ||
| 독성 | LD50 orally in rats: 10 mg/kg (Hayes) | ||
| IDLA | 25 mg/m3(as CN) | ||
| 기존화학 물질 | KE-29092 | ||
| 유해화학물질 필터링 | 97-1-90 | ||
| 함량 및 규제정보 | 물질구분: 유독물질; 혼합물(제품)함량정보: 무기시안 화합물 및 이를 1% 이상 함유한 혼합물. 다만, 베를린청(Ferric ferrocyanide), 페로시안염(Ferrocyanide, salts), 페리시안염(Ferricyanide, salts) 및 그 중 하나를 함유한 혼합물은 제외 |
사이안화 칼륨 C화학적 특성, 용도, 생산
물성
사이안화칼륨은 설탕과 매우 유사하게 보이는 무색의 결정이며, 물에 대한 용해도와 유독성이 매우 높고 습한 상태에서는 가수분해를 통해 소량의 사이안화수소를 생성한다.개요
사이안화 칼륨은 KCN 화학식을 지니는 무기 화합물이다. 치사량은 0.20g으로 극소량을 섭취해도 사망할 수 있는 매우 강력한 독극물이다. 본래는 전기 도금을 위한 전해질로 사용된다.용도
대부분의 사이안화칼륨은 금광업, 유기합성, 전기 도금에 쓰이며, 일부는 보석을 도금하거나 버핑하는 데에 사용되기도 한다.또한, 사이안화칼륨은 곤충을 재빨리 죽여 손상을 최소화할 수 있기 때문에 곤충학자들은 표본을 만들 때 사이안화칼륨을 사용하기도 한다.개요
Potassium cyanide, KCN, is a cyanide salt that is found as a white, amorphous, deliquescent lump or crystalline mass with a faint odor of bitter almonds. It is soluble in water and has a specific gravity of 1.52. It is a poison that is absorbed through the skin. Target organs are the same as for sodium cyanide. Reaction with acids releases flammable and toxic hydrogen cyanide gas. The four-digit UN identification number is 1680. The NFPA 704 designation is health 3, flammability 0, and reactivity 0. The primary uses are in gold and silver ore extraction, insecticides, fumigants, and electroplating.화학적 성질
Potassium cyanide are white lumps, granular powder, or colorless solution. It may be shipped as capsules, tablets, or pellets. Toxic hydrogen cyanide gas released by potassium cyanide has a distinctive, weak bitter almond odor, but many people cannot detect it; the odor does not provide adequate warning of hazardous concentrations.용도
Potassium cyanide is a white granular salt made by the absorption of hydrogen cyanide in potassium hydroxide. It is soluble in both water and alcohol and a lethal poison. If mixed with acids it produces highly toxic hydrogen cyanide gas. This was the preferred fixing chemical for collodion positives because it contained no sulfides to darken the highlight silver. As a fixing agent cyanide was particularly effective. After dissolving the unexposed silver halides cyanide would also remove nonimage fog producing very clean shadow areas. Prolonged fixing would eventually remove image silver. Tincture of iodine was added to dilute solutions of potassium cyanide and used to remove unwanted non-image silver in photographic materials and to remove silver stains.생산 방법
Potassium cyanide was made by the Beilby process before the introduction of the neutralization or wet process. When made by the neutralization or wet process, it contains 99% KCN. Initially, potassium cyanide was used as a flux and later for electroplating, which was the single largest use in the 1990s. The demand for potassium cyanide was met by the ferrocyanide process until the latter part of the nineteenth century when the extraordinary demands of the gold mining industry for alkali cyanide resulted in the development of direct synthesis processes. When cheaper sodium cyanide became available, potassium cyanide was displaced in many uses.일반 설명
White amorphous lumps or a crystalline mass with a faint odor of bitter almonds. Density 1.52 g / cm3 Toxic by skin absorption through open wounds, by ingestion. Heating to decomposition produces toxic fumes. Used for gold and silver extraction, in chemical analysis, to make other chemicals, and as an insecticide.공기와 물의 반응
Deliquescent. Soluble in water. Dissolution releases some poisonous hydrogen cyanide gas. The amount is not hazardous except in an enclosed space. If the water is acidic, dangerous amounts of hydrogen cyanide form at once.반응 프로필
POTASSIUM CYANIDE is a basic salt and a reducing agent. Reacts with acids of all kinds to generate poisonous hydrogen cyanide gas. Can react violently with oxidizing agents: fusion with metal chlorates, perchlorates, nitrates, or nitrites can cause explosions [Bretherick 1979. p. 101]. A mixture with perchloryl fluoride may explode above 100°C. A mixture with nitrite salts may cause an explosion [Pieters 1957. p. 30]. Incompatible with iodine. Initiates the explosive decomposition of nitrogen trichloride.위험도
A poison as absorbed by skin.건강위험
POTASSIUM CYANIDE is classified as super toxic. Probable oral lethal dose in humans is less than 5 mg/kg or less than a taste (7 drops) for a 150 lb. person. It is an eye and skin irritant. Poisonous in very small quantities; a taste is lethal.화재위험
Contact with acid releases highly flammable hydrogen cyanide gas. Moisture may cause POTASSIUM CYANIDE to volatilize as hydrogen cyanide. When heated to decomposition, POTASSIUM CYANIDE emits very toxic fumes of cyanide and nitrogen oxides. Reacts with acids to produce hydrogen cyanide gas. Reacts with strong oxidizers such as nitrates and chlorates, nitrogen trichloride; perchloryl fluoride; sodium nitrate; acids; alkaloids; chloral hydrate; iodine. Avoid contact with acids.공업 용도
A white amorphous or crystalline solid of the composition KCN, potassium cyanide is employed for carbonizing steel for case hardening and for electroplating. For cyaniding steel the latter is immersed in a bath of molten cyanide and then quenched in water, or the cyanide is rubbed on the red-hot steel.Commercial potassium cyanide is likely to contain a proportion of sodium cyanide. Potassium ferrocyanide, or yellow prussiate of potash, can also be used for case-hardening steel. It has the compositionK4Fe(CN)6and comes in yellow crystals or powder. The nitrogen as well as the carbon enters the steel to form the hard case. Potassium ferricyanide, or red prussiate of potash, is a bright-red granular powder of the composition K3Fe(CN)6, used in photographic reducing solutions, in etching solutions, in blueprint paper, and in silvering mirrors. Redsol crystals is the name of this chemical for use as a reducer and mild oxidizing agent, or toner, for photography.
Safety Profile
A deadly human poison by ingestion. A experimental poison by ocular, subcutaneous, intravenous, intramuscular, and intraperitoneal routes. Experimental teratogenic and reproductive effects. Human systemic effects by ingestion: convulsions, pulse rate increase. Mutation data reported. Reacts with acids or acid fumes to liberate deadly HCN. When heated to decomposition it emits very toxic fumes of K2O , CN-, and NOx. See also CYANIDE.잠재적 노출
Used in electroplating, steel hardening; extraction of precious metals form ores; as a fumigant; in insecticides; a reagent in analytical chemistry운송 방법
UN1680 Potassium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Purification Methods
A saturated solution in H2O-ethanol (1:3) at 60o is filtered and cooled to room temperature. Absolute EtOH is added, with stirring, until crystallisation ceases. The solution is again allowed to cool to room temperature (during 2-3hours), then the crystals are filtered off, washed with absolute EtOH, and dried, first at 70-80o for 2-3hours, then at 105o for 2hours [Brown et al. J Phys Chem 66 2426 1962]. It has also been purified by melting in a vacuum and by zone refining. HIGHLY POISONOUS.비 호환성
A strong reducing agent; keep away from oxidizers. Potassium cyanide decomposes on contact with water, humidity, carbon dioxide, strong acids (such as hydrochloric, sulfuric, and nitric acids), and acid salts, producing highly toxic and highly flammable hydrogen cyanide gas. Potassium cyanide absorbs water from air (is hygroscopic or deliquescent); the aqueous solution is a strong base. Incompatible with organic anhydrides; isocyanates, alkylene oxides; epichlorohydrin, aldehydes, alcohols, glycols, phenols, cresols, caprolactum, strong oxidizers; nitrogen trichloride; sodium chlorate. Attacks aluminum, copper, zinc in the presence of moisture.폐기물 처리
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water.사이안화 칼륨 준비 용품 및 원자재
원자재
준비 용품
POTASSIUM DICYANOAURATE(I)
푸로인
(S)-MEPHENYTOIN
7-METHYLADENINE
1H-INDAZOLE-5-CARBONITRILE
2-AMINO-2-METHYL-PROPIONITRILE
2,2-디클로로아세트아미드
Molsidomine
푸릴
3-퀴놀린카르보니트릴
1-이소퀴놀린카보나이트릴
1-CYANO-2-BENZOYL-1,2-DIHYDROISOQUINOLINE
2-METHYLBENZOYL CYANIDE
4-(N-CYANOMETHYL-N-NITROSO)AMINOMORPHOLINE
2-NAPHTHALEN-1-YL-ETHYLAMINE
4-FLUORO-2-NITROBENZONITRILE
Benzoin
2-퀴놀린카르보니트릴
4-CHLORO-PYRIDINE-2-CARBONITRILE
2-AMINOPROPANENITRILE
CYCLOLEUCINOL
1-Methyl-4-nitro-1H-imidazole-5-carbonitrile
Boc-L-beta-Homoproline
DL-Valine
3-Morpholinosydnonimine hydrochloride
숙시노나이트릴
2-(1,5-Dimethyl-1H-pyrazol-3-yl)acetonitrile ,97%
2-METHYLGLUTARIC ACID
시안화은
골드 시안화 칼륨
2,4-피리딘디카보니트릴
탈륨
퀴날딘산
2-AMINO-3-METHYLBUTANENITRILE
5-(AMINOMETHYL)-5-METHYLPYRROLIDIN-2-ONE
3-Methoxy-2-methylbenzoic acid
2-CYANO-4-METHYLPYRIDINE
5-AMINO-PYRIMIDINE-2-CARBOXYLIC ACID
2-(4-BIPHENYL)ETHYLAMINE
Ethyl 2,3-dicyanopropionate
사이안화 칼륨 관련 검색:
싸이오사이안산 칼륨 니켈 칼륨 시안화 페로사이안화칼륨 헥사시아노철(II)산칼륨 사이안화 칼륨 칼륨 은 시안화물 포타슘 헥사시아노 코발트(III) 골드 시안화 칼륨 염료 청색 27 시아노겐 칼륨 페리시안화칼륨 칼륨 테트라사이아노진케이트 포타슘 카드뮴 시아나이드
IRIDIUM POTASSIUM CYANIDE,IRIDIUM(III) POTASSIUM CYANIDE, 99.99%
PLATINUM POTASSIUM CYANIDE
GOLD(I) POTASSIUM CYANIDE,GOLD POTASSIUM CYANIDE,gold(i) potassium cyanide, premion
POTASSIUM CYANIDE-13C