|
|
| | 1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID Basic information |
| Product Name: | 1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID | | Synonyms: | 1,4-bis-Boc-piperazine-2-carboxylic acid;1,4-DI-BOC-PIPERAZINE-2-(+/-)-CARBOXYLIC ACID;1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID;1-N-Boc-4-N-Boc-piperazine-2-carboxylic acid;Piperazine-1,2,4-tricarboxylic acid 1,4-di-tert-butyl ester;1,2,4-Piperazinetricarboxylic acid, 1,4-bis(1,1-dimethylethyl) ester;1,2,4-Piperazinetricarboxylic acid 1,4-bis(tert-butyl) ester;1,4-Di-Boc-piperazine-2-carboxylic acid, 97.5% | | CAS: | 181955-79-3 | | MF: | C15H26N2O6 | | MW: | 330.38 | | EINECS: | | | Product Categories: | | | Mol File: | 181955-79-3.mol | 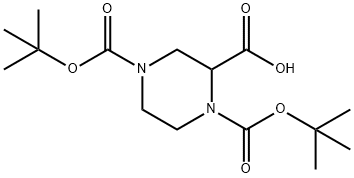 |
| | 1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID Chemical Properties |
| Melting point | 142-148°C | | Boiling point | 443.9±40.0 °C(Predicted) | | density | 1.200±0.06 g/cm3(Predicted) | | storage temp. | 2-8°C | | pka | 3.70±0.20(Predicted) | | form | solid | | Appearance | White to off-white Solid | | InChIKey | IIZGWFQKLVCLLA-UHFFFAOYSA-N |
| Hazard Codes | Xi | | Risk Statements | 36-43 | | Safety Statements | 26-36/37 | | WGK Germany | 3 | | HS Code | 29335990 |
| | 1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID Usage And Synthesis |
| Chemical Properties | White powder | | Synthesis | General procedure for the synthesis of 1,4-bis-BOC-2-piperazinecarboxylic acid from 2-piperazinecarboxylic acid and di-tert-butyl dicarbonate: In a 1 L round bottom flask, 15.76 g NaOH (394 mmol) was dissolved in 390 mL of water, followed by the addition of 20 g of 2-piperazinecarboxylic acid (98 mmol) in batches to obtain a colorless solution. A solution of 50 mL of Boc2O (215 mmol) dissolved in 1,4-dioxane was added slowly and dropwise while the temperature was maintained below +5 °C. Upon completion of the reaction, the mixture was cooled to +5 °C and the pH was subsequently adjusted to 3 with 3N HCl. The suspension was extracted with 300 mL of ethyl acetate (AcOEt), the organic phases were combined and dried over sodium sulfate. After vacuum evaporation, 22.7 g of white solid product was obtained. The aqueous phase was acidified with 3N HCl to pH=3 and then extracted with 2×100mL ethyl acetate (AcOEt), and the organic phases were similarly dried with sodium sulfate and then vacuum evaporated to give a second batch of 7.9g of product. The two batches of product were combined to give the final 1,4-bis-BOC-2-piperazinecarboxylic acid (30.6 g, 94% yield). The product was confirmed by 1H-NMR (400 MHz, DMSO-d6): δ 1.33 (18H, m), 2.70-4.50 (8H, m), 12.9 (1H, s); the mass spectrum (ES) showed m/z 330 [M+H]+. | | References | [1] Patent: WO2008/148853, 2008, A1. Location in patent: Page/Page column 157-158
[2] Patent: US2014/288045, 2014, A1. Location in patent: Paragraph 0531
[3] Angewandte Chemie - International Edition, 2018,
[4] Angew. Chem., 2018, vol. 130, # 44, p. 14768 - 14773,6
[5] Bioorganic and Medicinal Chemistry Letters, 2003, vol. 13, # 23, p. 4241 - 4244 |
| | 1,4-BIS(N-BOC)PIPERAZINE-2-CARBOXYLIC ACID Preparation Products And Raw materials |
|