|
|
| | 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid Basic information |
| Product Name: | 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid | | Synonyms: | 3-Bromo-1-(3-Chloro-2-Pyridinyl)-1H-Pyrazole;3-Bromo-1-(3-chloro-2-pyridyl)pyrazole-5-carboxylic Acid;1H-Pyrazole-5-carboxylic acid, 3-bromo-1-(3-chloro-2-pyridinyl)-;3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid ISO 9001:2015 REACH;3-bromo-1-(3-chloro-2-pyridinyl)-1H-Pyrazole-5-carboxylic ac...;cas 500011-86-9 95%;3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid 95%;Intermediate of Chlorantraniliprole (Coragen) | | CAS: | 500011-86-9 | | MF: | C9H5BrClN3O2 | | MW: | 302.51 | | EINECS: | 481-150-8 | | Product Categories: | | | Mol File: | 500011-86-9.mol | 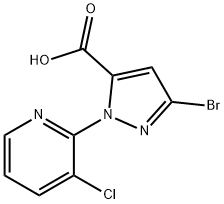 |
| | 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid Chemical Properties |
| Melting point | 197-200 °C | | Boiling point | 477.4±45.0 °C(Predicted) | | density | 1.92±0.1 g/cm3(Predicted) | | vapor pressure | 0Pa at 20℃ | | storage temp. | 2-8°C | | solubility | DMSO (Slightly), Methanol (Slightly) | | form | Solid | | pka | 2.39±0.36(Predicted) | | color | White to Light Brown | | Water Solubility | 8.84g/L at 20.3℃ | | InChI | InChI=1S/C9H5BrClN3O2/c10-7-4-6(9(15)16)14(13-7)8-5(11)2-1-3-12-8/h1-4H,(H,15,16) | | InChIKey | FORBXGROTPOMEH-UHFFFAOYSA-N | | SMILES | N1(C2=NC=CC=C2Cl)C(C(O)=O)=CC(Br)=N1 | | LogP | 1.5 at 25℃ |
| | 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid Usage And Synthesis |
| Uses | 3-Bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid is a useful building block and has been used in the synthesis of the pesticide Cyclaniliprole. | | Flammability and Explosibility | Not classified | | Synthesis | At -78 °C, 2-(3-bromo-1H-pyrazol-1-yl)-3-chloropyridine (3.04 g, 11.8 mmol) was dissolved in anhydrous THF (25 mL), keeping the reaction temperature below -71 °C. LDA solution (6 mL, 2M) was added slowly and dropwise. The reaction mixture was stirred at -78 °C for 15 min and then bubbled with carbon dioxide gas for 10 min. Subsequently, the reaction system was slowly warmed to -57°C and continued to -20°C. Upon completion of the reaction, the reaction was quenched with water. The reaction mixture was concentrated and dissolved in water (100 mL) and ether (80 mL), and an aqueous NaOH solution (1N, 2 mL) was added. The aqueous phase was washed with ether and acidified with aqueous HCl (2N). The precipitated solid was filtered, washed with water and dried to give 3-bromo-1-(3-chloro-2-pyridinyl)-1H-pyrazole-5-carboxylic acid (2 g, 59% yield) as a brown solid. | | References | [1] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 22, p. 6274 - 6279
[2] Patent: CN105669643, 2016, A. Location in patent: Paragraph 0072; 0075; 0076
[3] Patent: WO2006/68669, 2006, A1. Location in patent: Page/Page column 19; 25
[4] Patent: WO2003/106427, 2003, A2. Location in patent: Page 18-19
[5] Patent: WO2004/67528, 2004, A1. Location in patent: Page 27 |
| | 3-BroMo-1-(3-chloropyridin-2-yl)-1H-pyrazole-5-carboxylic acid Preparation Products And Raw materials |
|