|
ChemicalBook Optimization Suppliers |
|
| 化学名: | ソルビン酸 | | 英語化学名: | Sorbic acid | | 别名: | 2,4-Hexadienoic acid, (2E,4E)-;(e,e)-4-hexadienoicacid;2,4-Hexadienoicacid,(E,E)-;2e,4e-hexadienoicacid;4-Hexadienoicacid,(E,E)-2;2-PROPENYL ACRYLIC ACID;1,3-Pentadiene-1-carboxylic acid;ACIDUM SORBICUM | | CAS番号: | 110-44-1 | | 分子式: | C6H8O2 | | 分子量: | 112.13 | | EINECS: | 203-768-7 | | カテゴリ情報: | Antibiotics by Application;Antibiotics N-S;Preservatives and Disinfectants;Antibiotics;Food and Feed Additive;Food & Feed ADDITIVES;Fatty & Aliphatic Acids, Esters, Alcohols & Derivatives;Organic acids;FOOD ADDITIVES;Antibiotics A to Z;Antifungal;Biochemicals and Reagents;Building Blocks;C6;Carbonyl Compounds;Carboxylic Acids;Chemical Synthesis;Fatty Acids and conjugates;Food additive.;Fatty Acyls;Lipids;Organic Building Blocks;Others;Polyunsaturated;Spectrum of Activity;Unsaturated Fatty Acids and Derivatives;bc0001;110-44-1 | | Mol File: | 110-44-1.mol | 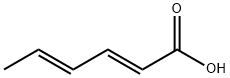 |
| 融点 | 132-135 °C (lit.) | | 沸点 | 228°C | | 比重(密度) | 1.2 g/cm3 at 20 °C | | 蒸気圧 | 0.01 mm Hg ( 20 °C) | | FEMA | 3921 | 2,4-HEXADIENOIC ACID, (E,E)- | | 屈折率 | 1.4600 (estimate) | | 闪点 | 127 °C | | 貯蔵温度 | 2-8°C | | 溶解性 | ethanol: 0.1 g/mL, clear | | 外見 | Crystalline Powder | | 酸解離定数(Pka) | 4.76(at 25℃) | | 色 | White or cream-white | | PH | 3.3 (1.6g/l, H2O, 20°C) | | 臭い (Odor) | bland | | 水溶解度 | 1.6 g/L (20 ºC) | | Merck | 14,8721 | | JECFA Number | 1176 | | BRN | 1741831 | | 安定性: | Material saturated with this acid may ignite spontaneously. Incompatible with strong oxidizing agents. May be light sensitive. | | InChIKey | WSWCOQWTEOXDQX-MQQKCMAXSA-N | | LogP | 1.32 at 20℃ | | CAS データベース | 110-44-1(CAS DataBase Reference) | | NISTの化学物質情報 | 2,4-Hexadienoic acid, (E,E)-(110-44-1) | | EPAの化学物質情報 | Sorbic acid (110-44-1) |
| | ソルビン酸 Usage And Synthesis |
| 外観 | 白色〜ほとんど白色, 結晶〜結晶性粉末 | | 定義 | 本品は、次の化学式で表される有機酸である。 | | 溶解性 | エタノール及びジエチルエーテルに溶け、水にほとんど溶けない。水に微溶 (30℃で0.25%)。エタノールに12.5%, メタノールに20%可溶。 | | 解説 | ソルビン酸,白色の針状晶.融点134.5 ℃,沸点228 ℃(分解).エタノール,エーテル,アセトンに可溶,水に難溶.かび,酵母,好気性菌に対して発育阻止作用があり,保存料,防腐剤として食品に用いられる.合成樹脂原料などにも用いられる.LD50 10.5 g/kg(ラット,経口).
森北出版「化学辞典(第2版)
| | 用途 | 防腐剤、保存料 | | 用途 | 中性または酸性で抗カビ力がすぐれ、特定食品の抗カビ剤に用いられる。また合成樹脂の原料にもなる。 | | 製法 | ソルビン酸,天然には,ナナカマドSorbus commixta H.の果実中に存在する.クロトンアルデヒドとマロン酸のピリジン中での縮合,あるいはアセトアルデヒド3分子から(順次アルドール結合と脱水を経て)誘導される.ヘキサジエナールの空気酸化でも得られ,市販品はtrans-trans形である. | | 化粧品の成分用途 | 防腐剤、香料 | | 効能 | 抗菌性保存剤 | | 毒性 | LD50 10.5 g/kg(ラット,経口). | | 使用上の注意 | 重合しやすい。 | | 説明 | Sorbic acid, also known as herbal tea acid, 2,4-hexadienoic acid,
2-propenyl acrylic acid, with molecular formula C6H8O2, is a food
additive that has inhibitory effects on many fungi such as yeast and
mold. It is also used in animal feed, cosmetics, pharmaceuticals,
packaging materials and rubber additives. | | 化学的特性 | White, crystalline solid. Slightly soluble in water and
many organic solvents. Combustible. | | 化学的特性 | (E,E)-2,4-Hexadienoic acid has a characteristic odor. | | 来歴 | Sorbic acid is a white crystalline solid first isolated in 1859 by hydrolysis of the oil distilled from unripened mountain-ash berries. The name is derived from the scientific term for the rowan tree, Sorbus aucuparia Linne, which is the parent plant of the mountain ash. Sorbic acid was first synthesized in 1900. Interest in this compound was minimal until independent researchers, E. Mueller of Germany and C.M. Gooding of the United States, discovered its antimicrobial effect in 1939 and 1940, respectively. Early interest in manufacturing sorbic acid centered around its use as a tung oil replacement when tung oil supplies were curtailed in the United States during World War II. High manufacturing costs prohibited expanded use until its approval as a food preservative in 1953. Sorbic acid is widely used in foods having a pH of 6.5 or below, where control of bacteria, molds, and yeasts is essential for obtaining safe and economical storage life. | | 使用 | Sorbic Acid is an naturally occurring organic compound first isolated from unripe berries. Sorbic acid has been used as a food preservative and as an inhibitor of Clostridium Botulinum bacteria in mea
t products in order to reduce the amount of nitrites which produce carcinogenic nitroamines. | | 使用 | Mold and yeast inhibitor. Fungistatic agent for foods, especially cheeses. To improve the characteristics of drying oils. In alkyd type coatings to improve gloss. To improve milling characteristics of cold rubber. See also Potassium Sorbate. | | 使用 | Sorbic Acid is a preservative that is effective against yeasts and molds.
it is effective over a broad ph range up to ph 6.5, being ineffective
above ph 7.0. it is a white, free-flowing powder which is slightly
soluble in water with a solubility of 0.16 g in 100 ml of water at
20°c. its solubility in water increases with increasing temperatures,
although it is not recommended in foods that are pasteurized
because it breaks down at high temperatures. the salts are potas-
sium, calcium, and sodium sorbate. it is used in cheese, jelly, bever-
ages, syrup, and pickles. typical usage levels range from 0.05 to
0.10%. | | 使用 | sorbic acid is a broad-spectrum, non-toxic preservative against molds and yeasts with moderate sensitizing potential in leave-on cosmetics. It is used in concentrations of 0.1 to 0.3 percent, and its activity is dependent on the formulation’s pH. Sorbic acid is used as a replacement for glycerin in emulsions, ointments, and various cosmetic creams. It is obtained from the berries of the tree commonly known as mountain ash and rowan, and can also be produced synthetically. Sorbic acid can cause irritation. | | 定義 | ChEBI: A sorbic acid having trans-double bonds at positions 2 and 4; a food preservative that can induce cutaneous vasodilation and stinging upon topical application to humans. It is the most thermodynamically stable of the four possible geometri
isomers possible, as well as the one with the highest antimicrobial activity. | | 反応性 | The chemical reactivity of sorbic acid is determined by the conjugated double bonds and the carboxyl group.
Sorbic acid is brominated faster than other olefinic acids. Reaction with hydrogen chloride gives predominately 5-chloro-3-hexenoic acid. Reactions with amines at high temperatures under pressure lead to mixtures of dehydro-2-piperidinones. A yellow crystalline complex is formed from sorbic acid and iron tricarbonyl. Similar coordination occurs also in the presence of other di- and trivalent metals. Reduction of the double bonds can produce various hexenoic acid mixtures. | | Biotechnological Production | Today, sorbic acid is produced solely by chemical synthesis. However, fermentation
and chemical synthesis might be combined to develop a new production
route for sorbic acid . In a first step, glucose would be converted to triacetic
acid lactone by fermentation. It has been shown that triacetic acid lactone can be
produced by genetically modified E. coli and S. cerevisiae strains. After a
separation from the fermentation broth, triacetic acid lactone would be transformed
into butyl sorbate in a multistage catalyst system (catalysis-hydrogenation
and solid acid catalysis). Then, butyl sorbate would be purified and hydrolyzed to
sorbic acid. Different scenarios are analyzed to evaluate the economic feasibility of
such a production process . | | Synthesis Reference(s) | Chemistry Letters, 10, p. 1289, 1981
Organic Syntheses, Coll. Vol. 3, p. 783, 1955
Tetrahedron Letters, 22, p. 69, 1981 DOI: 10.1016/0040-4039(81)80043-3 | | 一般的な説明 | White powder or crystals. Melting point 134.5°C. Slightly acidic and astringent taste with a faint odor. | | 空気と水の反応 | Soluble in hot water [Handbook of Chemistry and Physics]. May be sensitive to exposure to air and heat. The dust may become explosive, particularly when mixed with free-radical initiators or oxidizing agents. . | | 反応プロフィール | Sorbic acid may discolor on exposure to light. Can react with oxidizing agents. Also incompatible with bases and reducing agents. The dust may become explosive, particularly when mixed with free-radical initiators or oxidizing agents . | | 火災危険 | Sorbic acid is combustible. | | Biochem/physiol Actions | Sorbic acid can be used to inhibit bacterial, yeast and fungal sulfhydryl enzymes by inhibiting amino acid uptake. | | 毒性学 | Sorbic acid and its salts have broad-spectrum activity against yeast and molds, but are less active against bacteria. The antimicrobial action of sorbic acid was discovered independently in the United States and Germany in 1939, and since the mid-1950s sorbates have been increasingly used as preservatives. Sorbates generally have been found superior to benzoate for preservation of margarine, fish, cheese, bread, and cake. Sorbic acid and its potassium salts are used in low concentrations to control mold and yeast growth in cheese products, some fish and meat products, fresh fruits, vegetables, fruit beverages, baked foods, pickles, and wines. Sorbic acid is practically nontoxic. Table 10.4 shows acute toxicity of sorbic acid and its potassium salt. Animal studies have not shown obvious problems in tests performed with large doses for longer time periods. When sorbic acid (40 mg/kg/day) was injected directly into the stomach of male and female mice for 20 months, no differences were observed in survival rates, growth rates, or appetite between the injected mice and the control. When the dose was increased to 80 mg/kg/day for three additional months, however, some growth inhibition was observed. When potassium sorbate (1 and 2% in feed) was fed to dogs for three months, no pathological abnormalities were observed. This evidence indicates that the subacute toxicity of sorbic acid is negligible.
As a relatively new food additive, sorbate has been subject to stringent
toxicity-testing requirements. It may well be the most intensively studied
of all chemical food preservatives. In 90-day feeding studies in rats and dogs
and a lifetime feeding study in rats, a 5% dietary level of sorbates procured
no observable adverse effects. However, at a 10% dietary level in a 120-
day feeding study, rats showed increased growth and increased liver weight.
This has been attributed to the caloric value of sorbate at these high dietary
levels since it can act as a substrate for normal catabolic metabolism in
mammals. Sorbates are not mutagenic or tumorigenic, and as noted previously,
no reproductive toxicity has been observed. | | 安全性プロファイル | Moderately toxic by intraperitoneal and subcutaneous routes. Mildly toxic by ingestion. Experimental reproductive effects. A severe human and experimental skin irritant. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. Combustible when exposed to heat or flame; can react with oxidizing materials. To fight fire, use water. When heated to decomposition it emits acrid smoke and irritating fumes. | | 発がん性 | Wistar rats (six males) given subcutaneous
injections of 2mg sorbic acid, in 0.5mL of arachis
oil twice weekly for 65 weeks developed local sarcomas
. The first tumor was observed at 82 weeks.
Similar findings were also observed in follow-up studies
. However, six Wistar rats maintained on
drinking water containing 10 mg of sorbic acid/100mL
drinking water for 64 weeks did not develop tumors.
Tumors also were not observed inWistar rats (50 of each sex)
on diets that contained 40 mg/kg/day of sorbic acid for 18
months or in 25 male and female cross-bred white mice
after administration of 40 mg/kg/day for 17 months.
Mice fed a diet containing 15% sorbic acid for 88 weeks
exhibited a high incidence of hepatoma. Furthermore,
the glutathione level in the livers of the mice that ingested
15% sorbic acid decreased to 40% of the amount found in
controls after a 3-month feeding period; this low level was
maintained until the end of the experiments at 12 months.
There was a close correlation between the extent of depletion
of the glutathione level in the liver and the concentration
of sorbic acid added to the diet. In the same strain of mice fed
a diet containing 15% sorbic acid for up to 6 months, the
acidic fraction of an ether extract showed slight mutagenic
activity in an Ames test with Salmonella typhimurium TA98
in the presence of a liver 9000-g supernatant.
Consequently, the hepatomas that developed in mice fed a
15% sorbic acid diet were considered to be induced both by
the chronic depletion of the hepatic glutathione and by the
gradual production of various promutagens in the intestine,
which were absorbed and metabolically activated by the
liver. | | 貯蔵 | +4°C | | 純化方法 | Crystallise the acid from water. Dry it air or in a desiccator over P2O5. [Beilstein 2 IV 1701.] |
|