|
ChemicalBook Optimization Suppliers |
|
| | ネロリドール (cis-, trans-混合物) 製品概要 |
| 化学名: | ネロリドール (cis-, trans-混合物) | | 英語化学名: | Nerolidol | | 别名: | TRANS-3,7,11-TRIMETHYL-1,6,10-DODECATRIEN-3-OL;(CIS AND TRANS)-NEROLIDOL;FEMA 2772;3-HYDROXY-3,7,11-TRIMETHYL-1,6,10-DODECATRIENE,3,7,11-TRIMETHYL-1,6,10-DODECATRIEN-3-OL;10-dodecatrien-3-ol,3,7,11-trimethyl-6;3,7,11-trimethyl-1,6,10-dodecatrien-3-ol (nerolidol);3,7,11-Trimethyl-1,6,10-dodecatriene-3-ol;3,7,11-trimethyl-dodeca-1,6,10-trien-3-ol | | CAS番号: | 7212-44-4 | | 分子式: | C15H26O | | 分子量: | 222.37 | | EINECS: | 230-597-5 | | カテゴリ情報: | Biochemistry;Terpenes;Terpenes (Others);Chamaemelum nobile (Chamomile tea);Panax ginseng;Acyclic;Alkenes;Building Blocks;Chemical Synthesis;Citrus aurantium (Seville orange);Elettaria Cardamomum (Cardamom);Humulus lupulus (Hops);Nutrition Research;Ocimum basilicum (Basil);Organic Building Blocks;Phytochemicals by Plant (Food/Spice/Herb);bc0001 | | Mol File: | 7212-44-4.mol | 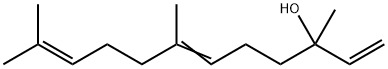 |
| | ネロリドール (cis-, trans-混合物) 物理性質 |
| 主な危険性 | Xi,N | | Rフレーズ | 36/37/38-50/53 | | Sフレーズ | 26-36-61-60 | | RIDADR | UN 3082 9 / PGIII | | WGK Germany | 2 | | RTECS 番号 | JR4977000 | | F | 10-23 | | 自然発火温度 | 230 °C | | TSCA | Yes | | HSコード | 29052290 | | 毒性 | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 5000 mg/kg |
| | ネロリドール (cis-, trans-混合物) Usage And Synthesis |
| 外観 | 無色~うすい黄色、液体 | | 用途 | 花、木を想起させる香気を持ち、ライラックやジャスミンなどのフレグランスの調合原料、石鹸や洗剤の香料として用いられる。ファルネソールやビタミンE、Kの中間体ともなる。 | | 使用上の注意 | アルゴン封入 | | 説明 | Nerolidol has a faint, floral odor similar to rose and apple. It is an unusually sweet, fresh, tenacious odor. The natural product can be dextro- or levo-rotatory, whereas the synthetic product is optically inactive; the double bond at positions 6-7 accounts for the cis- and trans-forms. | | 化学的特性 | Nerolidol is the sesquiterpene
analog of linalool. Because of the double bond at the 6-position, it exists as (6Z)- and (6E)- isomers, each forming an enantiomeric pair,
since the carbon atom in the 3-position is asymmetric.
Nerolidol is a component of many essential oils. (3S,6E)-(+)-nerolidol occurs in cabreuva oil.
Synthetic nerolidol consists of a mixture of racemic (6Z)- and (6E)-nerolidol
and is a colorless liquid with a long-lasting, mild floral odor.
Industrial synthesis of nerolidol starts with linalool, which is converted into
geranylacetone by using diketene, ethyl acetoacetate, or isopropenyl methyl
ether, analogously to the synthesis of 6-methyl-5-hepten-2-one from 2-methyl-
3-buten-2-ol. Addition of acetylene and partial hydrogenation of the resultant
dehydronerolidol produces a mixture of (6Z)- and (6E)-nerolidol racemates.Nerolidol is used as a base note in many delicate floral odor complexes. It is also
an intermediate in the production of vitamins E and K1. | | 化学的特性 | Clear slightly yellow liquid | | 化学的特性 | Nerolidol has a faint, fresh, unusually sweet, tenacious, floral odor similar to rose and appe | | 天然物の起源 | Reported found in over 25 natural sources, including the essential oils of neroli, ylang-ylang, Peru balsam; in
currant aroma; also reported in Dalbergia sissoo (60%, dl-), vervain, the distillation water of petitgrain bigarade, Helicrysum itali�cum, Myrocarpus frondosus and Myrocarpus fastigiatus (80%, d-), Tolu balsam, Acacia farnesiana, orange flower water, Paraguay
petitgrain, jasmine, Melaleuca smithii and Melaleuca viridiflora. Also reported found in citrus peel oils and juices, guava, straw�berry, peppermint and spearmint oil, pepper, milk powder, hop oil, beer, cognac, white wine, green tea, beans, mushroom, carda�mom, rice, dill, corn oil, basil, wort, myrtle leaf, maté and mastic gum oil. | | 使用 | Nerolidol is one of the main component in the essential oils from fresh aerial parts of Thymus ciliatus (Lamiaceae). Also, it is a terpene that displays high potency on the contractility of cardiac muscle in guinea pig left atrium. A potential and effective treatment for malaria. | | 使用 | anti-ulcer, insect antifeedant | | 定義 | ChEBI: Nerolidol is a farnesane sesquiterpenoid that is dodeca-1,6,10-triene which carries methyl groups at positions 3, 7 and 11 and a hydroxy group at position 3. It is a natural product that is present in various flowers and plants with a floral odor. Chemically, it exists in two geometric isomers, trans and cis forms. It is widely used in cosmetics (e.g. shampoos and perfumes), in non-cosmetic products (e.g. detergents and cleansers) and also as a food flavoring agent. It has a role as a flavouring agent, a cosmetic, a pheromone, a neuroprotective agent, an antifungal agent, an anti-inflammatory agent, an antihypertensive agent, an antioxidant, a volatile oil component, an insect attractant and a herbicide. It is a farnesane sesquiterpenoid, a tertiary allylic alcohol and a volatile organic compound. | | Aroma threshold values | Detection: 10 ppb to 10 ppm | | Taste threshold values | Taste characteristics at 25 ppm: green, floral, woody with fruity, citrus and melon nuances | | 一般的な説明 | Nerolidol is a naturally occurring sesquiterpene found in the essential oils. | | 燃焼性と爆発性 | Non flammable | | 合成 | The natural product can be dextro- or levorotatory, whereas the synthetic product is optically inactive; the double bond at
position 6 to 7 accounts for the cis- and trans-forms. |
|