- 6-AMINOISOQUINOLINE
-

- $1.10 / 1g
-
2025-11-18
- CAS:23687-26-5
- Min. Order: 1g
- Purity: 99.00%
- Supply Ability: 100 Tons
- 6-AMINOISOQUINOLINE
-
- $0.00 / 1kg
-
2025-04-04
- CAS:23687-26-5
- Min. Order: 1kg
- Purity: 98%
- Supply Ability: 1Ton
- 6-AMINOISOQUINOLINE
-

- $20.00 / 1kg
-
2023-08-09
- CAS:23687-26-5
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 50000tons
|
| | 6-AMINOISOQUINOLINE Basic information |
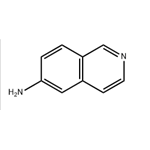
| Product Name: | 6-AMINOISOQUINOLINE | | Synonyms: | 6-AMINOISOQUINOLINE;6-aminoisoquanoline;6-Isoquinolinamine;Isoquinoline-6-amine;Isoquinolin-6-ylamine;Isoquinolin-6-amine, 6-Amino-2-azanaphthalene;Isoquinoline,6-amino- (7CI,8CI);Aminoisoquinoline | | CAS: | 23687-26-5 | | MF: | C9H8N2 | | MW: | 144.17 | | EINECS: | 639-440-9 | | Product Categories: | Building Blocks;Isoquinoline | | Mol File: | 23687-26-5.mol | 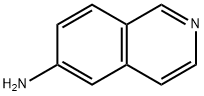 |
| | 6-AMINOISOQUINOLINE Chemical Properties |
| Melting point | 211-212 °C(Solv: benzene (71-43-2)) | | Boiling point | 343.1±15.0 °C(Predicted) | | density | 1.210±0.06 g/cm3(Predicted) | | storage temp. | Keep in dark place,Inert atmosphere,Room temperature | | solubility | DMF (Slightly), DMSO (Sparingly), Methanol (Slightly) | | pka | 7.10±0.10(Predicted) | | form | solid | | color | Light yellow to yellow | | InChI | InChI=1S/C9H8N2/c10-9-2-1-8-6-11-4-3-7(8)5-9/h1-6H,10H2 | | InChIKey | NGFCTYXFMDWFRQ-UHFFFAOYSA-N | | SMILES | C1C2=C(C=C(N)C=C2)C=CN=1 |
| Hazard Codes | Xn | | Risk Statements | 22-36 | | Safety Statements | 26 | | HS Code | 2933499090 |
| | 6-AMINOISOQUINOLINE Usage And Synthesis |
| Description | 6-Aminoisoquinoline is an organosynthetic intermediate used in the preparation of Netarsudil, which is a drug used in the treatment and management of glaucoma and high intraocular pressure. | | Uses | 6-Aminoisoquinoline is a reactant used in the preparation of N-[{2-(4-methylpiperidin-1-yl)-6-(trifluoromethyl)-pyridin-3-yl}methyl] N''-(6,6-fused heterocyclic) ureas as highly potent TRPV1 antagonists. | | Synthesis | General procedure for the synthesis of 6-aminoisoquinoline from 6-bromoisoquinoline: 17.2 g of 6-bromoisoquinoline (see WO 2008/077553), 200 mL of 28% ammonia solution and 10.8 g of copper (II) sulfate pentahydrate were placed in an autoclave and sealed. The mixture was stirred and reacted at 190 °C for 6 hours. After completion of the reaction, it was cooled to room temperature and the reaction solution was poured into 250 mL of 10% aqueous sodium hydroxide solution and extracted with ethyl acetate (100 mL each time, 5 times). The organic phases were combined, dried with anhydrous sodium sulfate, filtered and concentrated. The resulting crude product was suspended with dichloromethane and filtered to give 10.2 g of light brown crystalline 6-aminoisoquinoline in 85% yield.1H-NMR spectrum (CDCl3, δ ppm): 5.54 (broad single peak, 2H), 6.58 (single peak, 1H), 7.00 (double peak, J = 9.0 Hz, 1H), 7.35 (double peak, J = 5.5 Hz, 1H), 7.75 (double peak, J = 5.5 Hz, 1H). 7.75 (double peak, J = 9.0 Hz, 1H), 8.32 (double peak, J = 5.5 Hz, 1H), 8.98 (single peak, 1H). | | References | [1] Patent: US2012/35159, 2012, A1. Location in patent: Page/Page column 8 |
| | 6-AMINOISOQUINOLINE Preparation Products And Raw materials |
|