|
ChemicalBook Optimization Suppliers |
|
| 融点 | 223-225°C | | 貯蔵温度 | Inert atmosphere,Store in freezer, under -20°C | | 溶解性 | Soluble in DMSO (up to 18 mg/ml with warming). | | 外見 | Yellow powder. | | 酸解離定数(Pka) | pKa (25°): 5.42 | | 色 | White or off-white | | 安定性: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 3 months. | | InChI | InChI=1S/C22H23N3O4.ClH/c1-4-16-6-5-7-17(12-16)25-22-18-13-20(28-10-8-26-2)21(29-11-9-27-3)14-19(18)23-15-24-22;/h1,5-7,12-15H,8-11H2,2-3H3,(H,23,24,25);1H | | InChIKey | GTTBEUCJPZQMDZ-UHFFFAOYSA-N | | SMILES | C12C=C(OCCOC)C(OCCOC)=CC=1N=CN=C2NC1=CC=CC(=C1)C#C.Cl | | CAS データベース | 183319-69-9(CAS DataBase Reference) |
| Sフレーズ | 24/25 | | HSコード | 29335990 |
| | エルロチニブ塩酸塩 (TARCEVA) Usage And Synthesis |
| 外観 | 白色~うすい褐色、結晶性粉末~粉末 | | 溶解性 | ジメチルスルホキシドに溶け、アセトン及び水にほとんど溶けない。 | | 用途 | 上皮成長因子受容体(EGFR)
チロシンキナーゼの阻害剤です。EGFR 遺伝
子変異による、腫瘍細胞の増殖を抑制する作
用を示します。 | | 効能 | 抗悪性腫瘍薬, 受容体チロシンキナーゼ阻害薬 | | 商品名 | タルセバ (中外製薬); タルセバ (中外製薬) | | 説明 | Erlotinib, launched as once daily oral treatment for patients with
non-small-cell lung cancer (NSCLC), is an inhibitor of the epidermal growth factor
receptor (EGFR) tyrosine kinase, and it is the second small-molecule drug to
be marketed with this mechanism of action. Both erlotinib and its predecessor,
gefitinib, are members of the anilinoquinazoline class of tyrosine kinase inhibitors.
They compete with the binding of ATP to the intracellular tyrosine kinase domain
of EGFR, thereby inhibiting receptor autophosphorylation and blocking downstream
signal transduction. Erlotinib is prepared by the condensation of 3-ethynylaniline
with 4-chloro-6,7-bis(2-methoxyethoxy)quinazoline, which is a key
intermediate obtained in five synthetic steps starting from ethyl 3,4-
dihydroxybenzoate. In vitro, Erlotinib inhibits purified human EGFR tyrosine
kinase with an IC50 of 2 nM and blocks EGFR autophosphorylation in cellular
assays with an IC50 of 20nM. Treatment of human colon cancer cells with erlotinib
was associated with growth inhibition, G1 cell cycle arrest, and apoptosis.
Oral administration of erlotinib in athymic mice produced potent antitumor effects
with an ED50 of 9.2 mg/kg/day for HN5 head and neck xenografts and 14 mg/
kg/day for A431 epidermoid xenografts. The absorption of Erlotinib following oral
dosing is approximately 60%. Food greatly enhances the absorption allowing for
almost 100% bioavailability of the dose. The time to reach peak plasma levels of the drug is about 4 hours, and the half-life is approximately 36 hours. Steady-state drug
levels are reached in 7 to 8 days. Erlotinib has high protein binding (93%) and has
an apparent volume of distribution of 232 L. It is metabolized primarily by
CYP3A4 and to a lesser extent by CYP1A2 and CYP1A1. The drug is mainly
excreted in the feces with less than 9% of the dose found in the urine. Erlotinib is
labeled for the treatment of patients with locally advanced or metastatic NSCLC
who have failed one or more previous chemotherapy regimens. The recommended
dosage is 150 mg daily until disease progression is detected. In a randomized, double
blind, placebo-controlled trial involving 731 patients, 150 mg/day oral dose of
erlotinib resulted in a median overall survival of 6.7 months compared with 4.7
months in the placebo group (p<0.001). Progression-free survival was 9.9 weeks
and 7.9 weeks in the erlotinib and placebo groups, respectively (p<0.001). Survival
at one year was 31.2% in the erlotinib group versus 21.5% in the placebo group.
The use of erlotinib showed greater benefit in patients with EGFR positive tumors
and in those who never smoked. The most common adverse events reported in
clinical trials were rash (9%) and diarrhea (6%). Elevations in liver function
tests were also seen; however, these effects were mainly transient or associated with
liver metastases. As previously noted for gefitinib, erlotinib is also shown to
lack any clinical benefit in concurrent administration with platinum-based chemotherapy. | | 化学的特性 | Off-White Solid | | Originator | Pfizer (US) | | 使用 | Erlotinib hydrochloride (V), a quinazoline derived small
molecule inhibitor of epidermal growth factor receptor
(EDGFR) tyrosine kinase, was approved in November,
2004, for the treatment of advanced or metastatic non-smallcell
lung cancer. It belongs to the same class as gefitinib,another quinazoline approved for treatment of advanced lung
cancer, but with improved pharmacokinetic properties. The molecule was originated by Pfizer and development
initiated in collaboration with OSI, which assumed full
rights to the drug when Pfizer merged with Warner Lambert.
Subsequently, Genentech/Roche went into licensing
agreement with OSI to develop and market the drug in the
US and Worldwide. | | 使用 | Erlotinib HCl (OSI-744) is an EGFR inhibitor with IC50 of 2 nM, >1000-fold more sensitive for EGFR than human c-Src or v-Abl. Phase 3. | | 使用 | Selective epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor. Antineoplastic | | 定義 | ChEBI: A quinazoline hydrochloride compound having a (3-ethynylphenyl)amino group at the 4-position and two 2-methoxyethoxy groups at the 6- and 7-positions. | | brand name | Tarceva
(OSI). | | 一般的な説明 | Erlotinib is available as 25-, 100-, and 150-mg tablets fororal administration and is used after failure of first-linetherapy in metastatic NSCLC and as first-line therapy incombination with gemcitabine in the treatment of metastaticpancreatic cancer, and in treating malignant gliomas.The structural similarity to gefitnib imparts similar pharmacokineticbehavior with bioavailability of 60% and proteinbinding of 93%. The agent is extensively metabolizedprimarily by CYP3A4. Three major metabolic pathwayshave been identified, involving oxidative-O-demethylationof the side chains followed by further oxidation to give thecarboxlic acids, oxidation of the acetylene functionalityto give a carboxylic acid, and aromatic hydroxylation ofthe phenyl ring para to the electron-donating nitrogen. Themetabolites are primarily eliminated in the feces, and theterminal half-life is 36 hours.The major toxicities seenwith the agent are dose-limiting skin rash and diarrhea.Other common adverse effects include shortness of breath,fatigue, and nausea. | | 合成 | The synthesis of this agent is
based on the original patent and is shown in the Scheme. The 3,4-dihydroxy benzoate 31 was reacted with
bromoethyl methyl ether in the presence of potassium
carbonate and tetrabutyl ammonium iodide to give 32 in
93% yield. Nitration followed by hydrogenation provided 34
in 88% yield, which was then cyclized in formamide with
ammonium formate to provide quinazolone 35. Subsequent
reaction with oxalyl chloride gave quinazoline chloride 36,
which was then reacted with 3-ethynyl aniline (37) in
isopropanol in the presence of pyridine to give the desired
product erlotinib, which was isolated as the HCl salt (V).
An alternate synthesis, that used protected 3-trimethylsilyl
ethynyl aniline to couple to the quinazoline chloride 36, has
also been published. 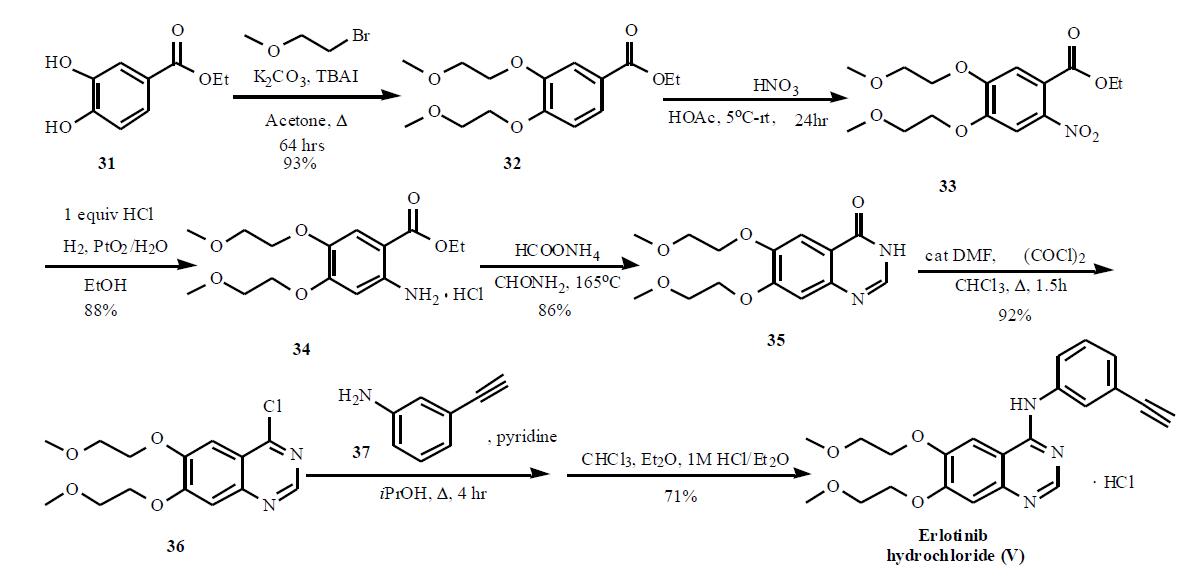
| | target | HER1/EGFR | | 貯蔵 | Store at -20°C | | 参考文献 | 1) Moyer et al. (1997), Induction of apoptosis and cell cycle arrest by CP-358,774, an inhibitor of epidermal growth factor tyrosine kinases; Cancer Res., 57 4838
2) Li et al. (2007), Erlotinib effectively inhibits JAK2V617F activity and polycythemia vera cell growth; J. Biol. Chem., 282 3428
3) Wood et al. (2004), A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells; Cancer Res., 64 6652
4) Greve et al. (2015), The pan-HDAC inhibitor panobinostat acts as a sensitizer for erlotinib activity in EGFR-mutated and –wildtype non-small cell lung cancer cells; BMC Cancer, 15 947
5) Minquet et al. (2016), Targeted therapies for treatment of non-small cell lung cancer-Recent advances and future perspectives; Int. J. Cancer, 138 2549 |
|