MIBEFRADIL DIHYDROCHLORIDE manufacturers
- Mibefradil
-

- $15.00 / 1KG
-
2021-07-13
- CAS:116644-53-2
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
- Mibefradil
-

- $15.00 / 1KG
-
2021-07-10
- CAS:116644-53-2
- Min. Order: 1KG
- Purity: 99%+ HPLC
- Supply Ability: Monthly supply of 1 ton
|
| | MIBEFRADIL DIHYDROCHLORIDE Basic information |
| Product Name: | MIBEFRADIL DIHYDROCHLORIDE | | Synonyms: | C07222;(1S,2S)-2-[2-[[3-(1H-Benzimidazol-2yl)propyl]methylamino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-(1-methylethyl)-2-naphthalenylmethoxyacetoacetatedihydrochloride;Acetic acid, Methoxy-, 2-[2-[[3-(1H-benziMidazol-2-yl)propyl]MethylaMino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-(1-Methylethyl)-2-naphthalenylester, (1S-cis)-;(1S,2S)-2-[2-[[3-(2-Benzimidazolyl)propyl]methylamino]ethyl]-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphthyl methoxyacetate;(1S,2S)-2-[2-[[3-(1H-BENZIMIDAZOL-2YL)PROPYL]METHYLAMINO]ETHYL]-6-FLUORO-1,2,3,4-TETRAHYDRO-1-(1-METHYLETHYL)-2-NAPHTHALENYL METHOXYACETOACETATE DIHYDROCHLORIDE;(1S,2S)-2-[2[[3-(2-BENZIMIDAZOLYLPROPYL]METHYLAMINO]ETHYL]-6-FLUORO-1,2,3,4-TETRAHYDRO-1-ISOPROPYL-2-NAPHTHYL METHOXYACETATE DIHYDROCHLORIDE;RO 40-5967;MIBEFRADIL DIHYDROCHLORIDE | | CAS: | 116644-53-2 | | MF: | C29H38FN3O3 | | MW: | 495.63 | | EINECS: | | | Product Categories: | | | Mol File: | 116644-53-2.mol | 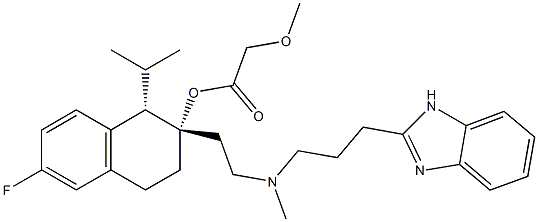 |
| | MIBEFRADIL DIHYDROCHLORIDE Chemical Properties |
| Melting point | 125 - 130°C | | Boiling point | 647.6±55.0 °C(Predicted) | | density | 1.18±0.1 g/cm3(Predicted) | | storage temp. | Desiccate at RT | | solubility | DMSO (Slightly), Methanol (Slightly) | | pka | 11.93±0.10(Predicted) | | form | Solid | | color | Off-White |
| | MIBEFRADIL DIHYDROCHLORIDE Usage And Synthesis |
| Originator | Posicor,Roche Pharmaceuticals,USA | | Uses | Vasodilator. | | Uses | Mibefradil dihydrochloride is selectively blocks Ca2+?entry into cells by inhibiting T-type Ca2+?channels. | | Definition | ChEBI: Mibefradil is a member of tetralins. | | Manufacturing Process | To the solution of 5.35 g (28 mmol) [3-(1H-benzimidazol-2-
yl)propyl]methylamine in 12.5 mL toluene was added by syringe 12.5 mL
(11.42 g, 114 mmol) isopropenyl acetate. The reaction mixture was heated to
reflux temperature, and stirred at that temperature for 1.75 hours, with
reaction completion monitored by thin-layer chromatography (silica gel,
eluting with 70% ethyl acetate/30% methanol). The product, N-[3-(1H�benzimidazol-2-yl)propyl]-N-methylacetamide, was obtained in quantitative
yield.
Under a dry nitrogen atmosphere, a 2.5 molar solution of butyl lithium in
hexane, 8.4 mL (21 mmol) was added by syringe to 20 mL pentane. The
solution was cooled to 0°C and 2.75 mL (2.13 g, 21 mmol) diisopropylamine
was added by syringe over six min. The solution was warmed to 25°C and
stirred for three hours, then volatiles were removed in vacuo. THF, 20 mL,
was added via syringe to the residue, and the resulting yellow solution cooled
to 0°C. A solution of 2.42 g (10.5 mmol) N-[3-(1H-benzimidazol-2-yl)propyl]-
N-methylacetamide in 10 mL THF was added by syringe over 9 min. The
yellow solution was stirred for 15 min, then cooled to -78°C. (S)-6-Fluoro-1-
isopropyl-3,4-dihydro-1H-naphthalen-2-one, 2.166 g, 87.2% pure (97.6:2.4
S:R), in 2 mL toluene was added by syringe over 12 min, and a further 2 mL
toluene was used to complete the transfer. After stirring for two hours, the
viscous yellow mixture was added to 50 mL water at less than 10°C. The
suspension that formed was extracted with diethyl ether; and the extracts
were dried over anhydrous magnesium sulfate, filtered, and concentrated in
vacuo to afford 3.74 g of impure (1S,2S)-N-[3-(1H-benzimidazol-2-yl)propyl]-
2-(6-fluoro-2-hydroxy-1-isopropyl-1,2,3,4-tetrahydronaphthalen-2-yl)-N-
methylacetamide as a yellow foam. The foam was recrystallized from toluene,
yield of a colorless solid 2.69 g, melting point 132-138°C. This material may
be recrystallized a second time from toluene to remove residual (S)-6-fluoro-
1-isopropyl-3,4-dihydro-1H-naphthalen-2-one if necessary.
(1S,2S)-N-[3-(1H-Benzimidazol-2-yl)propyl]-2-(6-fluoro-2-hydroxy-1-
isopropyl-1,2,3,4-tetrahydronaphthalen-2-yl)-N-methylacetamidemay be
synthesized by another method:
To the mixture 22.7 g (0.54 mol) dry lithium chloride and 100 mL THF at -
15°C was added 160 mL 2 molar lithium diisopropylamide (0.32 mol) in
heptane/THF/ethylbenzene was added. Then a solution of 36.6 g (0.16 mol)
N-[3-(1H-benzimidazol-2-yl)-propyl]-N-methylacetamide in 140 mL toluene
was added, the solution was stirred for 2 hours, and a further 155 mL toluene
was added. (S)-6-Fluoro-1-isopropyl-3,4-dihydro-1H-naphthalen-2-one (29.9
g, 0.15 mol), in 15 mL toluene was added. After stirring at -10°C for 4 hours,
the resulting solution was added to 200 mL ice water. The pH of the resulting
mixture was adjusted to 7-8 by addition of a 71 g concentrated hydrochloric
acid. The organic layer washed with water, then the solvents removed under
reduced pressure to give 96 g of (1S,2S)-N-[3-(1H-benzimidazol-2-yl)propyl]-
2-(6-fluoro-2-hydroxy-1-isopropyl-1,2,3,4-tetrahydronaphthalen-2-yl)-N�methylacetamide as a brown oil. The product was crystallysed from toluene,
yield 45.3 g.
(1S,2S)-N-[3-(1H-Benzimidazol-2-yl)propyl]-2-(6-fluoro-2-hydroxy-1-
isopropyl-1,2,3,4-tetrahydronaphthalen-2-yl)-N-methylacetamide,20.22 g
(45.7 mmol), dissolved in 200 mL toluene at 40°C, was added by cannula
over 40 min at 0°C to a suspension of sodium bis(2-methoxyethoxy)aluminum
hydride in toluene, 40 mL (41.44 g suspension, 26.94 g sodium bis(2-
methoxyethoxy)aluminum hydride, 133 mmol). The mixture was stirred at
0°C for 15 min, then at 35-40°C for 3 hours. The mixture was cooled to 25°C
then added carefully to 70 g sodium hydroxide in 140 g ice. The resulting
suspension was warmed to 25°C over 30 min, and the phases were separated.
The aqueous phase was extracted with toluene; and the organic phase was
washed twice with 10% aqueous sodium hydroxide, once with water, then
once with saturated brine. The toluene phase was dried and concentrated in
vacuo to afford 20.61 g of (1S,2S)-2-[2-{[3-(1H-benzimidazol-2-
yl)propyl]methylmethylamino}ethyl]-6-fluoro-1-isopropyl-1,2,3,4-
tetrahydronaphthalen-2-ol as a colorless foam.
To the mixture of 41.0 g (1S,2S)-2-[2-{[3-(1H-benzimidazol-2-
yl)propyl]methylmethylamino}ethyl]-6-fluoro-1-isopropyl-1,2,3,4-
tetrahydronaphthalen-2-ol, 240 mL water, and 240 mL toluene were added
22.4 g potassium hydroxide, and the mixture heated to 45-50°C for one hour.
The resulting two-phase mixture was separated. To the organic phase was
added 39.4 g (4.0 eq.) potassium carbonate sesquihydrate; then a solution of
21.0 g (17.7 mL, 3.25 eq.) methoxyacetyl chloride in 33 mL toluene was
added over two hours at 25-30°C, and the resulting mixture stirred for an
additional 30 min. Water, 200 mL, was added to quench the reaction. The
organic phase, containing mibefradil as the free base was added an ethanol.
To the stirred mixture of mibefradil and ethanol was added at 20°C a solution
of 4.4 g of hydrogen chloride in 44.6 mL (35.0 g) ethanol. The mixture was
heated to 50°C and 1.0 mL water was added, followed by a solution of 3.4 mL
water in 332 mL methyl tert-butyl ether over one hour. The mixture was stirred for 3 hours. Mibefradil dihydrochloride crystals was seeded. A solution
of 0.6 mL water in 65 mL methyl tert-butyl ether was added over one hour,
and the mixture aged for a further 1.5 hours. The mixture was then cooled,
and the resulting slurry of mibefradil dihydrochloride was filtered; yield 95%. | | Brand name | Posicor (Hoffmann-LaRoche). | | Therapeutic Function | Coronary vasodilator | | Biological Activity | Ca 2+ channel blocker with moderate selectivity for T-type Ca 2+ channels displaying IC 50 values of 2.7 μ M and 18.6 μ M for T-type and L-type channels respectively. Antihypertensive agent. |
| | MIBEFRADIL DIHYDROCHLORIDE Preparation Products And Raw materials |
|