|
ChemicalBook Optimization Suppliers |
|
| 化学名: | アナストロゾール | | 英語化学名: | Anastrozole | | 别名: | ICI-D-1033;1-[3,5-DI-(1-METHYL-1-CYANO)-ETHYL]-BENZYL-1,2,4-TRIAZOLE;α,α,α’,α'-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile;2-[3-(2-Cyanopropan-2-yl)-5-(1,2,4-triazol-1-ylmethyl)phenyl]-2-methylpropanenitrile;α1,α1,α3,α3-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile;Anastrozole(AriMidex);Anastrozole (200 mg);2-[3-(1-cyano-1-Methylethyl)-5-(1H-1,2,4-triazol-1-ylMethyl)phenyl]-2-Methylpropanenitrile | | CAS番号: | 120511-73-1 | | 分子式: | C17H19N5 | | 分子量: | 293.37 | | EINECS: | 601-715-6 | | カテゴリ情報: | Inhibitors;Intermediates & Fine Chemicals;Pharmaceuticals;anti-neoplastic;Anastrozole;API;All Inhibitors;Antineoplastic;Active Pharmaceutical Ingredients;120511-73-1;11 | | Mol File: | 120511-73-1.mol | 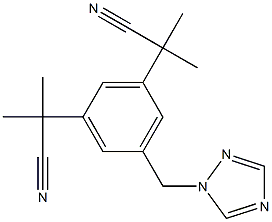 |
| 融点 | 81-82°C | | 沸点 | 469.7±55.0 °C(Predicted) | | 比重(密度) | 1.08±0.1 g/cm3(Predicted) | | 貯蔵温度 | room temp | | 溶解性 | DMSO: soluble40mg/mL | | 酸解離定数(Pka) | 2.62±0.10(Predicted) | | 外見 | solid | | BCS Class | 1 (LogP),
3 (CLogP) | | InChI | InChI=1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3 | | InChIKey | YBBLVLTVTVSKRW-UHFFFAOYSA-N | | SMILES | C(#N)C(C1=CC(CN2C=NC=N2)=CC(C(C#N)(C)C)=C1)(C)C | | CAS データベース | 120511-73-1(CAS DataBase Reference) |
| | アナストロゾール Usage And Synthesis |
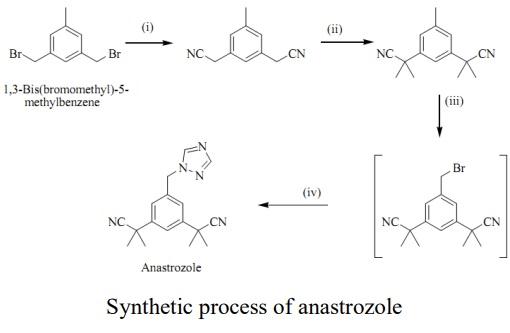
| 効能 | 抗悪性腫瘍薬, エストロゲン生合成阻害薬 | | 商品名 | アナストロゾール (第一三共エスファ); アリミデックス (アストラゼネカ) | | 説明 | Anastrozole(120511-73-1) was first approved for use in the United States in 1995 for the treatment of advanced breast cancer in post-menopausal women. Anastrozole is a highly potent and selective aromatase inhibitor. It is extremely potent in lowering circulating estradiol to undetectable levels in treated patients without altering other circulating hormones. The drug is reportedly well absorbed and tolerated following oral administration. | | 化学的特性 | Crystalline Solid. soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of anastrozole in these solvents is approximately 20, 13, and 14 mg/ml. | | Originator | Zeneca (United Kingdom) | | 使用 | Anastrozole is a nonsteroidal inhibitor of aromatase which effectively blocks estrogen synthesis in postmenopausal women and is used as therapy of estrogen receptor positive breast cancer. Anastrozole has been associated with a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent liver injury. | | 製造方法 | synthesis of anastrozole can be realized in four steps based on 3,5-bis(bromomethyl)toluene. Starting with a S N 2 displacement using potassium nitrile and tetrabutylammonium bromide as a phase transfer catalyst to give bis-nitrile compound. Bis-nitrile compound formed undergoes deprotonation with NaH and methylated afterwards with methyl iodide to give bis-dimethyated product.Product undergoes radical substitution reaction following the Wohl-Ziegler reaction using N-bromosuccinamide and benzoyl peroxide as the radical initiator.In the final step, benzylbromide undergoes SN2 displacement with sodium triazole to give anastrozole.
 | | 主な応用 | Anastrozole (aromatase inhibitor) has been used:
as a positive control in DNA fragmentation (ladder) assay
to investigate its effects along with extra virgin olive oil and its major fatty acid component (omega-9 OA) in estrogen receptor positive mammary adenocarcinoma cells
to study its effects on viability, cell proliferation and apoptosis in Glioblastoma multiforme model in vivo | | 定義 | ChEBI: Anastrozole is a 1,2,4-triazole compound having a 3,5-bis(2-cyano-2-propyl)benzyl group at the 1-position. It has a role as an antineoplastic agent and an EC 1.14.14.14 (aromatase) inhibitor. It is a member of triazoles and a nitrile. | | brand name | Arimidex
(AstraZeneca). | | Therapeutic Function | Antitumor | | 一般的な説明 | Anastrozole(120511-73-1) is a non-steroidal and expensive drug marketed under the trade name Arimidex. It was the first specific aromatase inhibitor approved in theUnited States. It is indicated for first-line treatment of postmenopausalwomen with advanced or metastatic breast cancer,for second-line treatment of postmenopausal patientswith advanced breast cancer who have had disease progressionfollowing tamoxifen therapy, and for adjuvant treatmentof women with early breast cancer. Patients who did not respondto tamoxifen therapy rarely respond to anastrozole. | | 生物活性 | Potent and highly selective aromatase (CYP19) inhibitor (IC 50 = 15nM) that has no discernible effect on adrenocorticoid hormone synthesis. Reduces plasma estrogen levels and exhibits antitumor activity in vivo . Orally active. | | Biochem/physiol Actions | Anastrozole, which contains a triazole functional group, reversibly binds to the cytochrome P-450 component of aromatase. Binding interferes with the catalytic properties of aromatase, which results in inhibition of estrogen synthesis. | | 作用機序 | Anastrozole, a benzyltriazole derivative, competes with the natural s ubstrate for binding to the active site of the aromatase. The mechanism of enzyme inhibition resides in the coordination of the triazole ring with the hemeiron atom of the aromatase enzyme complex. This coordination ultimately prevents arom atization of androgens into estrogens and, therefore, deprives the tumor of estrogen. This effect is reversible. In the presence of anastrozole, estradiol levels are reduced to undetectable levels, with no adverse effects on levels of any other horm one, including cortisol and aldosterone. Maximal estrogen suppression is produced by a 1mg dose. Estrogen suppression is maintained for up to 6 days after discontinuing anastrozole. | | 薬物動態学 | Anastrozole is well absorbed orally, with biliary elimination as its primary route (85%) and an elimination half-life of approxim ately 50 hours. Approximately 60% of an oral dose is m etabolized in the liver by N-dealkylation, hydroxylation, and glucuronidation to inactive triazole metabolites. | | 臨床応用 | Anastrozole is a potent and highly selective, nonsteroidal aromatase inhibitor utilized in the treatment of advanced breast cancer that is horm one-responsive. It is considered to be second-line therapy (after tamoxifen) in the treatment of postm enopaus al breast cancer. | | 副作用 | The most common anastrozole side effects are related to lower estrogen levels in the body. They include hot flashes, nausea and vomiting, and mood changes. Anastrozole could cause your bones to thin, which raises your risk of osteoporosis. It can also cause high cholesterol. | | 薬物相互作用 | Potentially hazardous interactions with other drugs
Oestrogen-containing therapies: avoid concomitant
administration as would negate pharmacological
action.
Tamoxifen: avoid concomitant administration. | | 環境運命予測 | Anastrozole is classified as readily biodegradable and is moderately mobile in soils. The measured octanol-water partition coefficient is low, therefore anastrozole is not predicted to bioaccumulate in aquatic organisms. | | 代謝 | Anastrozole is extensively metabolised by postmenopausal
women with less than 10% of the dose excreted in the
urine unchanged within 72 hours of dosing. Metabolism
of anastrozole occurs by N-dealkylation, hydroxylation
and glucuronidation via CYP 3A4 and 3A5, and
UGT1A4. The metabolites are excreted primarily via the
urine. Triazole, the major metabolite in plasma, does not
inhibit aromatase. | | 貯蔵 | Store at RT | | 参考文献 | [1] DUKESM. The preclinical pharmacology of “Arimidex” (anastrozole; ZD1033)–a potent, selective aromatase inhibitor.[J]. Journal of Steroid Biochemistry and Molecular Biology, 1996. DOI:10.1016/0960-0760(96)00064-7.
[2] U B. Anastrozole: a new addition to the armamentarium against advanced breast cancer.[J]. American Journal of Clinical Oncology-Cancer Clinical Trials, 1998. DOI:10.1097/00000421-199804000-00014.
[3] L?NNINGP E DowsettM GeislerJ. Pharmacological and clinical profile of anastrozole.[J]. Breast Cancer Research and Treatment, 1998. DOI:10.1023/a:1006000806630.
[4] HOZUMIYASUO. Effects of anastrozole on lipid metabolism compared with tamoxifen in rats.[J]. Breast Cancer Research and Treatment, 2002. DOI:10.1023/a:1020571617274. |
|