|
ChemicalBook Optimization Suppliers |
|
| 融点 | 51-56 °C (lit.) | | 沸点 | 200 °C (lit.) | | 比重(密度) | 1.48 | | 蒸気密度 | 3.4 (vs air) | | 蒸気圧 | 0.16 mm Hg ( 20 °C) | | 屈折率 | 1.4688 (estimate) | | 闪点 | 218 °F | | 貯蔵温度 | Store below +30°C. | | 溶解性 | Chloroform (Slightly), Ethyl Acetate (Slightly) | | 酸解離定数(Pka) | 0[at 20 ℃] | | 外見 | powder | | 色 | White | | PH | 0.8 (550g/l, H2O, 20℃)Hydrolysis | | 臭い (Odor) | Mild acrid odor. | | 爆発限界(explosive limit) | 1.4-7.1%(V) | | 水溶解度 | 79 g/100 mL (25 ºC) | | Sensitive | Moisture Sensitive | | Merck | 14,5704 | | BRN | 106909 | | Henry's Law Constant | (atm?m3/mol):
Not applicable - reacts with water | | 暴露限界値 | NIOSH REL: TWA 1 ppm (0.25 mg/m3), IDLH 10 ppm; OSHA PEL: TWA
0.25 ppm; ACGIH TLV: TWA 0.25 ppm with an intended change of 0.1 ppm. | | Dielectric constant | 51.0(60℃) | | 安定性: | Stable. Combustible. Incompatible with water, strong oxidizing agents, alkali metals, strong bases, amines, most common metals, polymerization catalysts and accelerators. | | InChIKey | FPYJFEHAWHCUMM-UHFFFAOYSA-N | | LogP | -2.61 at 20℃ | | CAS データベース | 108-31-6(CAS DataBase Reference) | | NISTの化学物質情報 | 2,5-Furandione(108-31-6) | | EPAの化学物質情報 | Maleic anhydride (108-31-6) |
| | 無水マレイン酸 Usage And Synthesis |
| 外観 | 白色, 結晶~粉末又は塊又は粒状 | | 定義 | 本品は、次の化学式で表される有機化合物である。 | | 溶解性 | 水に可溶 (反応しマレイン酸を生成する)、エタノールに可溶(反応しマレイン酸エチルを生成する)。 | | 解説 | 無水マレイン酸,ムスイマレインサン,2,5-furandione.C4H2O3(98.06).工業的には,ベンゼンを酸化バナジウムあるいは酸化モリブデンの存在下に空気酸化すると得られる.実験室的には,マレイン酸あるいはリンゴ酸を脱水するか,アセチルマレン酸無水物を熱分解すると得られる.無色の針状結晶.融点52.8 ℃,沸点202 ℃,95 ℃(2.66 kPa).d1.48.昇華性.水,アセトン,酢酸エチル,クロロホルム,ベンゼンに可溶,四塩化炭素,石油エーテルに難溶.共役二重結合をもつ鎖状あるいは環状化合物と容易にディールス-アルダー反応を行う.還元すると無水コハク酸となる.また,加圧下でアンモニアあるいは種々のアミンと反応させると,アスパラギン酸あるいはそのN-置換体を生じる.不飽和ポリエステル樹脂および種々の共重合体の製造のほか,有機合成の中間体になる.皮膚や眼などについたときは十分に水で洗う. | | 用途 | 可塑剤、合成樹脂塗料 | | 用途 | 不飽和ポリエステル樹脂、テトラハイドロフラン、フマール酸、コハク酸、リンゴ酸、紙サイズ剤、合成樹脂塗料、塩ビ安定剤、可塑剤、農薬、皮なめし、界面活性剤 | | 化粧品の成分用途 | 人工爪剤 | | 特徴 | 無水マレイン酸は分子内に2重結合、および、2つのカルボニル基を有するため、反応性に富み、生分解性も良好な物質です。
| | 主な用途 | 無水マレイン酸は、ブタンまたはベンゼンなどのC4炭化水素を、酸化バナジウム系触媒の存在下で空気酸化して製造されます。加水分解によりマレイン酸、加アルコール分解によりエステルを生成します。
無水マレイン酸は食添用途から工業用途に至るまで、幅広い分野の原料として使用されています。
■ 合成樹脂原料(不飽和ポリエステル)
■ 塗料
■ 樹脂改質剤
■ 塩ビ安定剤
■ 食品添加剤(フマル酸、コハク酸、リンゴ酸)
■ 農薬
■ 紙サイズ剤
■ イミド類
■ 界面活性剤
■ 可塑剤(DOM、DBM、DEM)
■ その他(GBL、14BG、THF)
| | 使用上の注意 | 吸湿性あり | | 説明 | Maleic Anhydride (MAN) is an organic compound with the chemical formula C4H203. It is the acid anhydride of maleic acid and in its pure state it is a colourless or white solid with an acrid odour. Maleic anhydride is truly a remarkable molecule in that it possesses two types of chemical functionality making it uniquely useful in chemical synthesis and applications.

Maleic Anhydride is a multifunctional chemical intermediate with applications in several fields of chemical industry. Its major end use is as feedstock in the production of unsaturated polyester resins (UPR).
These resins are used both in glass-reinforced and in unreinforced applications. The UPR end uses includes a wide range of applications in construction, marine and automobile industries.
In addition, Maleic Anhydride can also be used as raw material in the production of 1,4-butanediol (BDO), gamma- butyrolactone and tetrahydrofuran (THF). It is important to highlight that BDO is one of the world's fastest growing chemicals in the last years. | | 化学的特性 | Maleic anhydride is colorless needles, white lumps, or pellets. Irritating, choking odor. Dissolves in water to produce maleic acid. Dissolves in ethanol and produces esters. | | 物理的性質 | White, hydroscopic crystals (usually shipped as briquettes). Odor threshold concentration is 0.32
ppm (quoted, Amoore and Hautala, 1983). | | 使用 | In the manufacture of polyester resins,
fumaric acid, agricultural pesticides, and alkyl
resins | | 使用 | Maleic Anhydride is heterocyclic compound used in the manufacture of unsaturated polyester resins. Maleic Anhydride has a wide range of other applications; it is used in synthetic tensides, insecticides, herbicides and fungicides. | | 使用 | In Diels-Alder syntheses (as a dienophile), manufacture of alkyd-type of resins, dye intermediates, pharmaceuticals, agricultural chemicals (maleic hydrazide, malathion), in copolymerization reactions. | | 製造方法 | To a flask equipped with a Dean-Stark trap, condenser, and mechanical stirrer is added 116 gm (1.0 mole) of maleic acid and 120 ml of tetrachloroethane. The contents are heated, the water (18 ml, 1.0 mole) distilled off as the azeotrope, and the residue distilled under reduced pressure to afford 87.7 gm (89.5%) of the anhydride, b.p. 82-84°C (15 mm), m.p. 53°C. The residue remaining in the flask consists of about 10 gm of fumaric acid, m.p. 287°C.
Fumaric and maleic acids both give maleic anhydride on heating. Fumaric acid must first be heated to a higher temperature to effect its conversion to maleic acid prior to its dehydration.
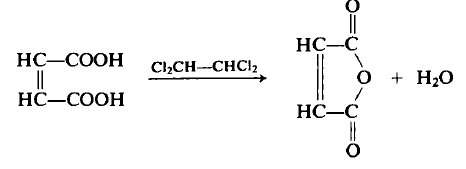 | | 定義 | ChEBI: Maleic anhydride is a cyclic dicarboxylic anhydride that is the cyclic anhydride of maleic acid. It has a role as an allergen. It is a cyclic dicarboxylic anhydride and a member of furans. | | 調製方法 | Maleic anhydride was traditionally manufactured by the oxidation of benzene or other aromatic compounds. As of 2006, only a few smaller plants continue to use benzene; due to rising benzene prices, most maleic anhydride plants now use n-butane as a feedstock.
In both cases, benzene and butane are fed into a stream of hot air, and the mixture is passed through a catalyst bed at high temperature. The ratio of air to hydrocarbon is controlled to prevent the mixture from catching on fire. Vanadium pentoxide and molybdenum trioxide are the catalysts used for the benzene route, whereas vanadium and phosphorus oxides are used for the butane route.
2 CH3CH2CH2CH3 + 7 O2 → 2 C2H2(CO)2O + 8 H2O. | | 反応性 | The chemistry of maleic anhydride is very rich, reflecting its ready availability and bifunctional reactivity. It hydrolyzes, producing maleic acid, cis-HOOC–CH=CH–COOH. With alcohols, the halfester is generated, e.g., cis-HOOC–CH=CH–COOCH3.
Maleic anhydride is a potent dienophile in Diels-Alder reactions. It is also a ligand for low-valent metal complexes, examples being Pt(PPh3)2(MA) and Fe(CO)4(MA).
Maleic anhydride dimerizes in a photochemical reaction to form cyclo butane tetra carboxylic dianhydride (CBTA). This compound is used in the production of polyimides and as an alignment film for liquid crystal displays. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 60, p. 6676, 1995 DOI: 10.1021/jo00126a013 | | 一般的な説明 | Colorless crystalline needles, flakes, pellets, rods, briquettes, lumps or a fused mass. Melts at 113°F. Shipped both as a solid and in the molten state. Vapors, fumes and dusts strong irritate the eyes, skin and mucous membranes. Flash point 218°F. Autoignition temperature 890°F. Used to make paints and plastics and other chemicals. | | 空気と水の反応 | Soluble in water. Reacts slowly with water to form maleic acid and heat. | | 反応プロフィール | Maleic anhydride react vigorously on contact with oxidizing materials. Reacts exothermically with water or steam. Undergoes violent exothermic decomposition reactions, producing carbon dioxide, in the presence of strong bases (sodium hydroxide, potassium hydroxide, calcium hydroxide), alkali metals (lithium, sodium, potassium), aliphatic amines (dimethylamine, trimethylamine), aromatic amines (pyridine, quinoline) at temperatures above 150° C [Vogler, C. A. et al., J. Chem. Eng. Data, 1963, 8, p. 620]. A 0.1% solution of pyridine (or other tertiary amine) in Maleic anhydride at 185°C gives an exothermic decomposition with rapid evolution of gas [Chem Eng. News 42(8); 41 1964]. Maleic anhydride is known as an excellent dienophile in the Diels-Alder reaction to produce phthalate ester derivatives. These reactions can be extremely violent, as in the case of 1-methylsilacyclopentadiene [J. Organomet., Chem., 1979, 179, c19]. Maleic anhydride undergoes a potentially explosive exothermic Diels-Alder reaction with 1-methylsilacyclopenta-2,4-diene at 150C [Barton, T. J., J. Organomet. Chem., 1979, 179, C19], and is considered an excellent dieneophile for Diels-alder reactions [Felthouse, Timothy R. et al. "Maleic anhydride , Maleic Acid, and Fumaric Acid." Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2005]. | | 危険性 | Irritant to tissue. Dermal and respiratory
sensitization. Questionable carcinogen. | | 健康ハザード | Inhalation causes coughing, sneezing, throat irritation. Skin contact causes irritation and redness. Vapors cause severe eye irritation; photophobia and double vision may occur. | | 火災危険 | Behavior in Fire: When heated above 300°F in the presence of various materials may generate heat and carbon dioxide. Will explode if confined. | | 燃焼性と爆発性 | Non flammable | | 安全性プロファイル | Poison by ingestion and
intraperitoneal routes. Moderately toxic by
skin contact. A corrosive irritant to eyes,
skin, and mucous membranes. Can cause
pulmonary edema. Questionable carcinogen
with experimental tumorigenic data.
Mutation data reported. A pesticide.
Combustible when exposed to heat or
flame; can react vigorously on contact with
oxidizing materials. Explosive in the form of
vapor when exposed to heat or flame.
Reacts with water or steam to produce heat.
Violent reaction with bases (e.g., sodmm
hydroxide, potassium hydroxide, calcium
hydroxide), dkah metals (e.g., sodium,
potassium), amines (e.g., dimethylamine,
triethylamine), lithium, pyridine. To fight
fire, use alcohol foam. Incompatible with
cations. When heated to decomposition
(above 150℃) it emits acrid smoke and
irritating fumes. See also ANHYDRIDES. | | 職業ばく露 | Maleic anhydride is used in unsaturated polyester resins; Agricultural chemical, and lubricating additives; in the manufacture of unsaturated polyester
resins; in the manufacture of fumaric acid; in alkyd resin manufacture; in the manufacture of pesticides e.g., malathion, maleic hydrazide, and captan). | | 応急処置 | If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. If victim is conscious, administerwater or milk. Do not induce vomiting. The symptoms ofasthma may be delayed for several hours and are aggravatedby physical effort. Rest and medical observation are highlyrecommended. Medical observation is recommended for24°48 h after breathing overexposure, as pulmonary edemamay be delayed. As first aid for pulmonary edema, a doctoror authorized paramedic may consider administering a corticosteroid spray. | | 貯蔵 | Color Code—White: Corrosive or Contact Hazard;Store separately in a corrosion-resistant location. Maleicanhydride must be stored to avoid contact with water andstrong oxidizers (such as chlorine and bromine), since violent reactions occur. Before entering confined space wherethis chemical may be present, check to make sure that anexplosive concentration does not exist. Sources of ignition,such as smoking and open flames, are prohibited where thischemical is handled, used, or stored. Metal containersinvolving the transfer of 5 gallons or more of this chemical should be grounded and bonded. Drums must be equippedwith self-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Wherever this chemical is used, handled, manufactured,or stored, use explosion-proof electrical equipment andfittings. | | 輸送方法 | UN2215 Maleic anhydride, Hazard class: 8; Labels: 8-Corrosive material.
Maleic Anhydride is commercialized and transported in the solid and molten forms. The molten Maleic Anhydride is transported at temperatures ranging from 60 to 80°C in well-insulated tank containers or road tankers provided with heating devices. In the solid form, it can be transported as pastilles, which are usually packed in polyethylene bags of 25 kg and transported either by rail tanker or by truck. | | 純化方法 | Crystallise it from *benzene, CHCl3, CH2Cl2 or CCl4. Sublime it under reduced pressure. [Skell et al. J Am Chem Soc 108 6300 1986, Beilstein 17 III/IV 5897, 17/11 V 55.] | | Toxicity evaluation | Maleic anhydride was described as having anticarcinogenic
properties, and some of the maleic copolymers can have biologic
activity by themselves, especially antitumor activity.
Information related to this compound is contradictory. Chromosomal
aberrations in cultured hamster cells but no mutagenicity
in in vitro tests in bacteria have been reported.
No effects on cholinesterase activity have been described
after exposure to maleic anhydride. | | 不和合性 | Reacts slowly with water (hydrolyzes) to
form maleic acid, a medium-strong acid. Dust may form
explosive mixture with air. Reacts with strong oxidizers,
oil, water, alkali metals; strong acids; strong bases. Violent
reaction with alkali metals and amines above 66�C.
Dangerous reaction with oxidizers, amines, alkali metals,
and hydroxides. Compounds of the carboxyl group react
with all bases, both inorganic and organic (i.e., amines)
releasing substantial heat, water and a salt that may be
harmful. Incompatible with arsenic compounds (releases
hydrogen cyanide gas), diazo compounds, dithiocarbamates, isocyanates, mercaptans, nitrides, and sulfides
(releasing heat, toxic, and possibly flammable gases),
thiosulfates and dithionites (releasing hydrogen sulfate
and oxides of sulfur) | | 廃棄物の処理 | Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform to EPA regulations governing
storage, transportation, treatment, and waste disposal.
Controlled incineration: care must be taken that complete
oxidation to nontoxic products occurs. |
|