|
ChemicalBook Optimization Suppliers |
| 名前: |
Alfa Aesar |
| 電話番号: |
400-6106006 |
| 電子メール: |
saleschina@alfa-asia.com |
|
| 化学名: | trans-アネトール | | 英語化学名: | trans-Anethole | | 别名: | trans-Anethole, 98+%, stab.;trans-Anethole, 98.5%;trans-Anethole;trans-Anethole, stabilized;(E)-1-METHOXY-4-(1-PROPENYL)BENZENE;1-methoxy-4-(1-propenyl)-,(E)-Benzene;anethole (E);Anistearoptene | | CAS番号: | 4180-23-8 | | 分子式: | C10H12O | | 分子量: | 148.2 | | EINECS: | 224-052-0 | | カテゴリ情報: | Inhibitors;PLAVIX | | Mol File: | 4180-23-8.mol | 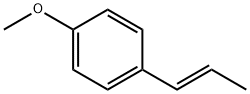 |
| 融点 | 20-21 °C(lit.) | | 沸点 | 234-237 °C(lit.) | | 比重(密度) | 0.988 g/mL at 25 °C(lit.) | | 蒸気圧 | 1.33 hPa (63 °C) | | FEMA | 2086 | TRANS-ANETHOLE | | 屈折率 | n20/D 1.561(lit.) | | 闪点 | 195 °F | | 貯蔵温度 | 2-8°C | | 溶解性 | Soluble in benzene, ethyl acetate, acetone, carbon disulfide. | | 外見 | Liquid After Melting | | 色 | Clear colorless to pale yellow | | PH | 7 (H2O) | | 臭い (Odor) | at 10.00 % in dipropylene glycol. sweet anise licorice mimosa | | においのタイプ | licorice | | 水溶解度 | practically insoluble | | Sensitive | Light Sensitive | | Merck | 14,643 | | JECFA Number | 217 | | BRN | 774229 | | 安定性: | Light sensitive | | InChIKey | RUVINXPYWBROJD-ONEGZZNKSA-N | | LogP | 3.388 | | CAS データベース | 4180-23-8(CAS DataBase Reference) | | NISTの化学物質情報 | Anethole(4180-23-8) | | EPAの化学物質情報 | (E)-Anethole (4180-23-8) |
| 主な危険性 | Xi,N | | Rフレーズ | 43-51/53 | | Sフレーズ | 36/37-61 | | RIDADR | UN 3077 9 / PGIII | | WGK Germany | 2 | | RTECS 番号 | BZ9275000 | | F | 8 | | TSCA | Yes | | HSコード | 29093090 | | 毒性 | LD50 orally in Rabbit: 2090 mg/kg LD50 dermal Rabbit > 5000 mg/kg |
| | trans-アネトール Usage And Synthesis |
| 外観 | 無色~ほとんど無色透明液体 | | 定義 | 本品は、次の化学式で表される芳香族エーテルである。 | | 化粧品の成分用途 | 変性剤、皮膚コンディショニング剤、香料、香味剤 | | 説明 | (E)-Anethol is a phenylpropanoid that has been found in P. anisum seed oil and has antifungal and antioxidant activity. It is active against fermentatively growing S. cerevisiae under hypoxic, but not normoxic, conditions (MIC = 100 μg/ml), and against C. parapsilosis when used at a concentration of 15% w/w. (E)-Anethol has antioxidant activity in a Trolox equivalent antioxidant capacity (TEAC) assay but does not scavenge 2,2-diphenyl-1-picrylhydrazel (DPPH) radicals in a cell-free assay. | | 化学的特性 | trans-anethole is a clear colorless to pale yellow liquid and has a characteristic anise, sweet, spicy, warm odor and corresponding sweet taste.

Anethole (1-methoxy-4-propenyl-benzene, isoestragole) is an alkoxypropenylbenzene derivative and an important favoring component of essential oils of more than 20 plant species. Essential oils from seeds of anise (Pimpinellaanisum L.), star anise (Illicium verum Hook.f), and sweet fennel (Foeniculum vulgare Mill. var. dulce) are the main sources used for the isolation of anethole. Two isomers of anethole occur in nature: E- or trans-anethole and Z-or cis-anethole. About 90 % of natural anethole is trans-isomer. Besides separation from natural essential oils, anethole is obtained using the rectification of crude sulfate turpentine and/or the organic synthesis starting from methylchavicol or anisole and propionic anhydride. Compared to natural compound, synthetic trans-anethole is impurified with higher amounts of cis-isomer. | | 天然物の起源 | Anethole is methyl ether of oestrone and has been found in fennel, aniseed, coriander, and many other volatile oils. | | 使用 | trans-Anethole is used to inhibits lung and forestomach carcinogenesis, used as carbon and energy supplement in the culture media of Pseudomonas putida strain. It is also used as used as a flavoring substance. | | 使用 | flavoring agent in food, dentifrices, etc.; in perfumery for soap, etc.; in pharmaceuticals as flavor; in photography and in
embedding materials in microscopy; some perfumery uses (fennei; absinthe; Hyacinth jacinthe; detergents; magnolia). Natural
occurrence: star anise | | 使用 | platelet aggregation inhibitor | | 使用 | expectorant, gastric stimulant, insecticide | | 製造方法 | By isomerization of estragole using alcoholic potassium hydroxide as agent (Arctander, 1969). | | 定義 | ChEBI: Anethole is a monomethoxybenzene that is methoxybenzene substituted by a prop-1-en-1-yl group at position 4. It has a role as a plant metabolite. | | 製造方法 | By esterification of p-cresol with methyl alcohol and with subsequent condensation with α-cetaldehyde (Perknis); the
most common method of preparation is from pine oil. By fractional distillation of the essential oils of anise, star anise, and fennel;
the anise essences contain an average of 85% anethole; fennel, from 60 to 70%. | | Taste threshold values | Taste characteristics at 10 ppm: sweet, anise, licorice and spicy with a lingering, sweet aftertaste. | | Synthesis Reference(s) | The Journal of Organic Chemistry, 50, p. 1797, 1985 DOI: 10.1021/jo00211a002
Tetrahedron, 24, p. 2183, 1968 DOI: 10.1016/0040-4020(68)88120-7 | | 一般的な説明 | trans-Anethole is a naturally occuring flavouring agent. It has insecticidal, larvicidal, and antimicrobial properties. | | Biochem/physiol Actions | Naturally occurring phenylpropene derivative that is estrogenic at lower concentrations and cytotoxic at higher concentrations to cancer cell lines. The cytotoxicity is related to the metabolism of trans-anethole to 4-hydroxy-1-propenylbenzene. | | 抗がん研究 | It is one of the major constituents of essential oil of fennel and anise and belongs tothe class of phenylpropenes. It has the capacity to block both inflammation andcarcinogenesis. It is an antioxidant and also a suppressor of NF-κB activation byIκBα degradation (Aggarwal and Shishodia 2004). | | 代謝 | Anethole is metabolized by oxidation of the propenyl group and is excreted as anisic acid (Williams, 1959). The metabolism of trans-anethole used in the preparation of anis-flavoured alcoholic beverages was studied in the rabbit and rat after iv and oral administration. It was excreted rapidly from the animal regardless of the method of administration. After iv injection it was found concentrated in the liver, lungs and brain; after oral administration, absorption was slight and most of it remained in the stomach. Ethyl alcohol has no effect on the metabolism (Le Bourhis, 1968). |
|