|
|
| | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine Basic information |
| Product Name: | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine | | Synonyms: | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine;(4,4-Dimethyl-cyclohexyl)-methanol;4-HydroxyMethyl-4-fluoro-...;4-HydroxyMethyl-4-fluoro-tetrahydro-2H-pyran;(4-Fluorotetrahydropyran-4-yl)methanol;(4-fluorooxan-4-yl)methanol;2H-Pyran-4-methanol, 4-fluorotetrahydro-;4-Fluorotetrahydropyran-4-methanol | | CAS: | 883442-46-4 | | MF: | C6H11FO2 | | MW: | 134.15 | | EINECS: | | | Product Categories: | | | Mol File: | 883442-46-4.mol | 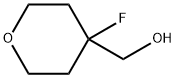 |
| | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine Chemical Properties |
| Boiling point | 198℃ | | density | 1.12 | | Fp | 100℃ | | storage temp. | 2-8°C | | Appearance | Colorless to light yellow Liquid |
| | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine Usage And Synthesis |
| Uses | (4-Fluorotetrahydro-2H-pyran-4-yl)methanol is a reactant used to prepare GPR40 agonists and beta secretase inhibitors. | | Synthesis | General procedure for the synthesis of (4-fluorotetrahydro-2H-pyran-4-yl)methanol from methyl 4-fluorotetrahydro-2H-pyran-4-carboxylate: 4-fluorotetrahydro-2H-pyran-4-carboxylic acid methyl ester (840 mg, 5.18 mmol) was dissolved in tetrahydrofuran (20 mL) under nitrogen protection and cooled to 0 °C. Subsequently, lithium aluminum hydride (394 mg, 10.36 mmol) was slowly added. The reaction mixture was stirred continuously at 0 °C for 3 hours. Upon completion of the reaction, saturated aqueous ammonium chloride solution (10 mL) was carefully added to quench the reaction. The reaction mixture was filtered and the filtrate was extracted with ethyl acetate (10 mL × 2). The organic layers were combined, dried with anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give the intermediate (4-fluorotetrahydro-2H-pyran-4-yl)methanol (550 mg, 4.10 mmol, 80% yield) as an oil, which was used directly in the next step of the reaction. The 1H NMR (CD3OD, 400 MHz) data of the intermediate were as follows: δ 3.81-3.85 (m, 2H), 3.70-3.76 (m, 2H), 3.64 (s, 1H), 3.59 (s, 1H), 1.67-1.90 (m, 4H). | | References | [1] Patent: WO2018/50684, 2018, A1. Location in patent: Page/Page column 68 |
| | (4-fluorotetrahydro-2H-pyran-4-yl)methanamine Preparation Products And Raw materials |
|