|
|
| | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate Basic information |
| Product Name: | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate | | Synonyms: | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate;methyl 3-hydroxy-4-(2-(trimethylsilyl)ethynyl)benzoate;Benzoic acid, 3-hydroxy-4-[2-(trimethylsilyl)ethynyl]-, methyl ester;Propanoicacid,6-methoxy-;Methyl 3-hydroxy-4-((trimethylsilyl)ethynyl)benzoate - [M56992];Lifitegrast Impurity 94 | | CAS: | 478169-68-5 | | MF: | C13H16O3Si | | MW: | 248.35 | | EINECS: | | | Product Categories: | | | Mol File: | 478169-68-5.mol | 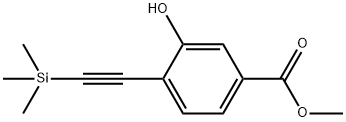 |
| | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate Chemical Properties |
| Boiling point | 332.2±42.0 °C(Predicted) | | density | 1.10±0.1 g/cm3(Predicted) | | storage temp. | Inert atmosphere,Room Temperature | | pka | 8.80±0.40(Predicted) | | Appearance | Light yellow to yellow Solid |
| | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate Usage And Synthesis |
| Synthesis | Methyl 3-hydroxy-4-iodobenzoate (5.22 g, 18.8 mmol) and trimethylsilylacetylene (3.71 mL, 26.3 mmol) were used as raw materials, which were added under nitrogen protection along with bis(triphenylphosphine)palladium dichloride (386 mg, 0.55 mmol) and cuprous iodide (54 mg, 0.28 mmol) in a dry THF (20 mL ) and CHCl3 (40 mL) in a solvent mixture. Subsequently, triethylamine (8.14 mL, 58.4 mmol) was added to the reaction system and the mixture was heated to 50 °C for 4 hours. After completion of the reaction, the reaction mixture was diluted with CHCl3 (60 mL), washed sequentially with 5% HCl (2 x 40 mL), and the organic phase was dried with MgSO4 and concentrated to give a brown oily solid (8.31 g). The crude product was purified by column chromatography (Biotage standard column, 90 g silica gel), eluting first with 10% EtOAc/hexane (1 L) and then with 15% EtOAc/hexane (1 L). The fraction containing the target product was collected and concentrated to give methyl 3-hydroxy-4-((trimethylsilyl)alkynyl)benzoate (4.22 g, 91% yield) as a yellow solid. High-resolution mass spectrometry (FAB) analysis results: C13H16O3Si [M+H]+ calculated value 249.0947, measured value 249.0947. | | References | [1] Journal of Medicinal Chemistry, 2006, vol. 49, # 14, p. 4425 - 4436
[2] Patent: US2003/232853, 2003, A1. Location in patent: Page 38
[3] Patent: US2003/73707, 2003, A1
[4] Patent: US2003/105089, 2003, A1 |
| | Methyl 3-hydroxy-4-((triMethylsilyl)ethynyl)benzoate Preparation Products And Raw materials |
|