|
ChemicalBook Optimization Suppliers |
|
| 化学名: | CB3715673 | | 英語化学名: | Citronellal | | 别名: | 2,3-Dihydrocitral;d-rhodinal;Levo-citronellal;3,7-DIMETHYL-6-OCTEN-1-AL;(+/-)-3,7-DIMETHYL-6-OCTENAL;(+/-)-CITRONELLAL;CITRONELLAL;CITRONELLAL 80 | | CAS番号: | 106-23-0 | | 分子式: | C10H18O | | 分子量: | 154.25 | | EINECS: | 203-376-6 | | カテゴリ情報: | Furans ,Coumarins | | Mol File: | 106-23-0.mol | 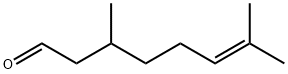 |
| 融点 | -16°C (estimate) | | 比旋光度 | D25 +11.50° | | 沸点 | 207 °C(lit.) | | 比重(密度) | 0.857 g/mL at 25 °C(lit.) | | 蒸気圧 | 14 hPa (88 °C) | | 屈折率 | n20/D 1.451(lit.) | | FEMA | 2307 | CITRONELLAL | | 闪点 | 169 °F | | 貯蔵温度 | Store below +30°C. | | 溶解性 | Chloroform (Soluble), Methanol (Sparingly) | | 外見 | Liquid | | 色 | Clear light yellow | | 比重 | 0.858 (20/4℃) | | PH | 7 (H2O) | | 臭い (Odor) | at 10.00 % in dipropylene glycol. sweet dry floral herbal waxy aldehydic citrus | | においのタイプ | floral | | 爆発限界(explosive limit) | 1.2-4.5%(V) | | 水溶解度 | Slightly miscible with water and ethanol. | | Sensitive | Air Sensitive | | Merck | 14,2329 | | JECFA Number | 1220 | | BRN | 1720789 | | 安定性: | Hygroscopic | | InChIKey | NEHNMFOYXAPHSD-UHFFFAOYSA-N | | LogP | 3.62 at 25℃ | | CAS データベース | 106-23-0(CAS DataBase Reference) | | NISTの化学物質情報 | 6-Octenal, 3,7-dimethyl-(106-23-0) | | EPAの化学物質情報 | Citronellal (106-23-0) |
| | CB3715673 Usage And Synthesis |
| 外観 | 無色〜わずかにうすい黄色, 澄明の液体 | | 定義 | 本品は、次の化学式で表されるテルペンアルデヒドである。 | | 溶解性 | 水に微溶, エタノール, エーテル, クロロホルムに可溶。エタノールに溶け、水にほとんど溶けない。 | | 解説 | シトロネラール,シトロネラ油には,(R)-(+)-シトロネラールが含まれている.還元すればシトロネロールを生じる.沸点47 ℃(0.13 kPa).[α]D+11.5°. 森北出版「化学辞典(第2版)
| | 用途 | 柑橘様香気を有する食品、化粧品香料に使用するが、アルカリ、空気、光に対する不安定さのために、単品として使われることは少ない。 | | 化粧品の成分用途 | 香料 | | 製造 | シトロネラール,非環状セスキテルペンアルデヒド.イネ科コウセンガヤChloris radiataから得られる. | | 化学的特性 | (?)-Citronellal occurs in Java citronella oil at a concentration of 35%. Racemic citronellal is the main constituent of E. citriodora
oil with a content of up to 85%.
Pure citronellal is a colorless liquid with a refreshing odor, reminiscent of
lemon balm. Upon catalytic hydrogenation, citronellal yields dihydrocitronellal,
citronellol, or dihydrocitronellol, depending on the reaction conditions. Protection
of the aldehyde group, followed by addition of water to the double bond in
the presence of mineral acids or ion-exchange resins results in the formation of
3,7-dimethyl-7-hydroxy-octan-l-al (hydroxydihydrocitronellal). Acid-catalyzed
cyclization to isopulegol is an important step in the synthesis of (?)-menthol. | | 化学的特性 | Ceylon citronella (C. nardus) and Java citronella (C. winterianus) are both perennial grasses growing more than 1 m
high. The herb is harvested two to three times a year in Ceylon. The freshly cut or partially dried herb is steam distilled. The plant
yields the largest amount of essential oil at about its third year of growth. Citronella is also cultivated and distilled in Java, Guatemala,
Taiwan, Hainan, Argentina and New Guinea. The Java-type essential oil is considered to be of superior quality over the Ceylon type.
Citronella has a characteristic citronella, rose- and lemon-like odor. The Council of Europe (CoE, 2000) has described Ceylon citronella
and Java Citronella separately. | | 化学的特性 | clear light yellow liquid | | 化学的特性 | Citronellal has an intense, lemon-, citronella-, rose-type odor. | | 天然物の起源 | The d-form of citronellal has been reported in the oil of citronella (Ceylon, Jammus, Kaschmis), in the oil from leaves of Barosma pulchella, in the oil from roots of Phebalium nudum and in the oils of Eucalyptus citriodora, Leptospermum citratum and Baeckea citriodora. The /-form is present in the oils of Backhousia citriodora var. A, E. citriodora, Litsea cubeba (fruits) and lemon�grass. Citronellal is generally present also in the oils of lemon, mandarin, Lavandula delphinensis, Ocimum canum f. citrata and many others (Fenarolis Handbook of Flavor Ingredients, 1971). | | 使用 | Citronellal is a flavoring agent that is a liquid, faintly yellow with an
intense odor resembling lemon, citronella, and rose. it is soluble in
alcohol and most fixed oils, slightly soluble in mineral oil and pro-
pylene glycol, and insoluble in water and glycerin. it is obtained by
chemical synthesis; the aldehyde may be obtained from natural oils,
such as citronella oil. it is also termed 3,7-dimethyl-6-octen-1-a1. | | 使用 | citronella is used primarily as a fragrance (perfuming and masking), it also has tonic properties. It is derived from the essential oil of the Cymbopogon nardus plant, and its constituents include geraniol (approximately 60 percent), citronellal, camphene, limonene, linalool, and borneol. | | 使用 | rac-Citronellal is a monoterpenoid and the major isolate in citronella oil. Citronella oil is an essential oil bearing insecticidal properties. rac-Citronellal is also often used as a fragrance ingred
ient. | | 製造方法 | Citronellal is still isolated from essential oils in considerable quantities;
it is also produced synthetically.
1) Isolation from essential oils:(+)-Citronellal is obtained from citronella oils
by fractional distillation. Racemic citronellal is isolated from E. citriodora oil;
when necessary, it is purified by using an addition compound, for example,
the bisulfite derivative.
2) Synthesis from geraniol or nerol: Racemic citronellal can be obtained by vaporphase
rearrangement of geraniol or nerol in the presence of, for example, a
barium-containing copper–chromium oxide catalyst.
3) Synthesis from citronellol: Racemic citronellal can also be obtained by dehydrogenation
of citronellol under reduced pressure with a copper chromite
catalyst.
4) Synthesis from citral: Selective hydrogenation of citral to citronellal can be
accomplished in the presence of a palladium catalyst in an alkaline alcoholic
reaction medium. A continuously operating process for the hydrogenation
on a palladium catalyst in the presence of trimethylamine has been developed.
5) Synthesis from myrcene: (+)- and (?)-Citronellal are available from myrcene
via geranyldiethylamine, which is enantioselectively isomerized to (+)- or (?)-citronellalenamine. Hydrolysis yields pure (+)- or (?)-citronellal; see monograph menthol. | | 定義 | ChEBI: A monoterpenoid, the main component of citronella oil which gives it its distinctive lemon aroma. | | 精油の成分 | Citronella oil contains a number of fragrant fractions of which citronellal, geraniol and citronellol are the
major components. Ceylon citronella oil contains 55 to 65% total acetylizable alcohols (calculated as citronellol) and 7 to 15% total
aldehyde (calculated as citronellal). The main constituents are geraniol (18 to 20%), citronellol (6.4 to 8.4%), citronellal (5 to 15%),
geranyl acetate (2%); limonene (9 to 11%) and methyl isoeugenol (7.2 to 11.3%). Other constituents are camphene, caryophyllene, linalool,
citral (neral and geranial), methylheapenone, methyleugenol, l-borneol, nerol, eugenol and farnesol.* Java citronella oil contains
not less than 35% alcohols (calculated as citronellol) and not less than 35% aldehydes (calculated as citronellal). The Java type appears
to have higher concentrations of citronellol (35%) and geraniol (21%) than does the Ceylon type (citronellol 10% and geraniol 18%). | | Aroma threshold values | Detection: 31 to 100 ppb | | Taste threshold values | Taste characteristics at 10 ppm: floral, green, rosy and citrus-lemon. | | Synthesis Reference(s) | Journal of the American Chemical Society, 108, p. 7314, 1986 DOI: 10.1021/ja00283a029
Tetrahedron Letters, 30, p. 5677, 1989 DOI: 10.1016/S0040-4039(00)76168-5
The Journal of Organic Chemistry, 49, p. 2279, 1984 DOI: 10.1021/jo00186a038 | | 一般的な説明 | (±)-Citronellal was studied for its fumigant antifungal activity against Pyricularia (Magnaporthe) grisea. | | 燃焼性と爆発性 | Non flammable | | 合成 | Can be prepared by chemical synthesis or by fractional distillation of natural oils, such as citronella. Industrially
prepared by hydrogenation of β-citronellol or by catalytic hydrogenation of citral; also in the laboratory by dehydration of
hydroxydihydrocitronellal. | | 代謝 | Feeding 50 g citronellal to rabbits was followed by the isolation of 13 g of a crystalline glucuronide, which proved to be p-menthane-3.8-diol-D-glucuronide. The citronellal appeared to have been cyclized and the glucuronide obtained was identical with that obtained on feeding p-men�thane-3,8-diol (menthoglycol) (Kühn & Low, 1938). However, evidence was produced to show that the cyclization was not, strictly speaking, a biological reaction, but a chemical one which took place in the stomach under the influence of the gastric hydrochloric acid. The conjugation of the menthoglycol with glucuronic acid was, of course, a purely biological reaction. It was found that on shaking 20 g citronellal with 200 ml 0-5% HCl for 48 hr at 37 C, 12 g menthoglycol was formed (Kühn & Low, 1938). |
|