|
ChemicalBook Optimization Suppliers |
|
| 化学名: | リモナバン | | 英語化学名: | Rimonabant | | 别名: | ACOMPLIA;RIMONABANT;RIMONABANT(ACOMPLIA,SR141716);5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-piperidinopyrazole-3-carboxamide;1H-Pyrazole-3-carboxamide, 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-;5-(4-Chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-N-1-piperidinyl-1H-pyrazole-3-carboxamide;A 281;Sr 141716 | | CAS番号: | 168273-06-1 | | 分子式: | C22H21Cl3N4O | | 分子量: | 463.79 | | EINECS: | 200-223-5 | | カテゴリ情報: | SR141716;Weight Loss | | Mol File: | 168273-06-1.mol | 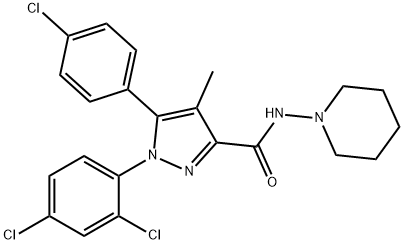 |
| 融点 | 154.7 °C | | 比重(密度) | 1.41±0.1 g/cm3(Predicted) | | 貯蔵温度 | RT | | 溶解性 | Soluble in DMSO (up to 20 mg/ml) or in Ethanol (up to 20 mg/ml). | | 酸解離定数(Pka) | 11.31±0.20(Predicted) | | 外見 | solid | | 色 | White | | 安定性: | Stable for 1 year from date of purchase as supplied. Protect from moisture. Solutions in DMSO or ethanol may be stored at -20°C for up to 3 months. | | CAS データベース | 168273-06-1(CAS DataBase Reference) |
| | リモナバン Usage And Synthesis |
| 効能 | 抗肥満薬, 禁煙補助薬, カンナビノイド受容体逆作動薬 | | 説明 | Rimonabant is a first-in-class drug launched as an oral treatment
for obesity, and its mechanism of action involves the selective antagonism
of cannabinoid type 1 (CB1) receptor. It is specifically indicated as an adjunct
to diet and exercise for the treatment of obese patients (body mass index
[BMI]≥30 kg/m2), or overweight patients (BMI>27 kg/m2) with associated risk
factors such as type 2 diabetes or dyslipidemia. Additionally, rimonabant is
currently under development as a treatment for nicotine dependence. The CB1
and CB2 receptors, along with their endogenous ligands, constitute the endocannabinoid
system. The CB1 receptor is expressed in the brain, adipose tissue,
and several peripheral organs; the CB2 receptor is predominantly expressed in
immune cells. Activation of the CB1 receptor in the CNS is associated with
appetite stimulation and the modulation of brain reward mechanism, whereas
activation in the periphery favors metabolic processes that lead to hepatic lipogenesis
and impaired glucose homeostasis. Rimonabant acts by selectively
blocking the action of central and peripheral CB1 receptors, thereby reducing
food intake and improving lipid and glucose metabolism. | | Originator | Sanofi-Synthelabo (France) | | 使用 | Rimonabant is a selective antagonist of CB1 with IC50 of 13.6 nM and EC50 of 17.3 nM in hCB1 transfected HEK 293 membrane | | 定義 | ChEBI: Rimonabant is a carbohydrazide obtained by formal condensation of the carboxy group of 5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxylic acid with the amino group of 1-aminopiperidine. It is a potent and selective cannabinoid receptor 1 (CB1R) antagonist. Besides its antagonistic properties, numerous studies have shown that, at micromolar concentrations rimonabant behaves as an inverse agonist at CB1 receptors. The drug was the first selective CB1 antagonist/inverse agonist introduced into clinical practice to treat obesity and metabolic-related disorders. It was later withdrawn from market due to CNS-related adverse effects including depression and suicidal ideation. It has a role as an anti-obesity agent, a CB1 receptor antagonist and an appetite depressant. It is a member of pyrazoles, a dichlorobenzene, a carbohydrazide, an amidopiperidine and a member of monochlorobenzenes. | | brand name | Acomplia (Sanofi-Synthe-labo). | | 合成 | The reported preparation of rimonabant,
both in small and large scale, is shown in the scheme. Lithium enolate formation of p-chlorophenyl ethyl ketone 54 with LiHMDS in THF at -78oC for 45 min followed
by reaction with diethyl oxalate at -78oC and warming
to room temperature over 16 h provided the lithium enolate
salt of the diketoester 55. Reaction of diketoester salt 55 with
2,4-dichlorophenyl hydrazine (56) in ethanol at room temperature
gave intermediate hydrazone 57 which is then cyclized
in refluxing acetic acid for 24 h to obtain pyrazole
ester 58. Hydrolysis of ester 58 with KOH in refluxing
methanol:water mixture gave acid 59 which was then converted
to the acid chloride 60 with thionyl chloride in refluxing
toluene in very good yield. On scale, the synthesis of the
acid chloride was performed in cyclohexane at 83oC. Reaction
of acid chloride 60 with 1-aminopiperidine (61) in the
presence of triethylamine at 0oC to room temperature over 3h
gave rimonabant (VIII) which was isolated as the HCl salt
by treating it with HCl in ether. 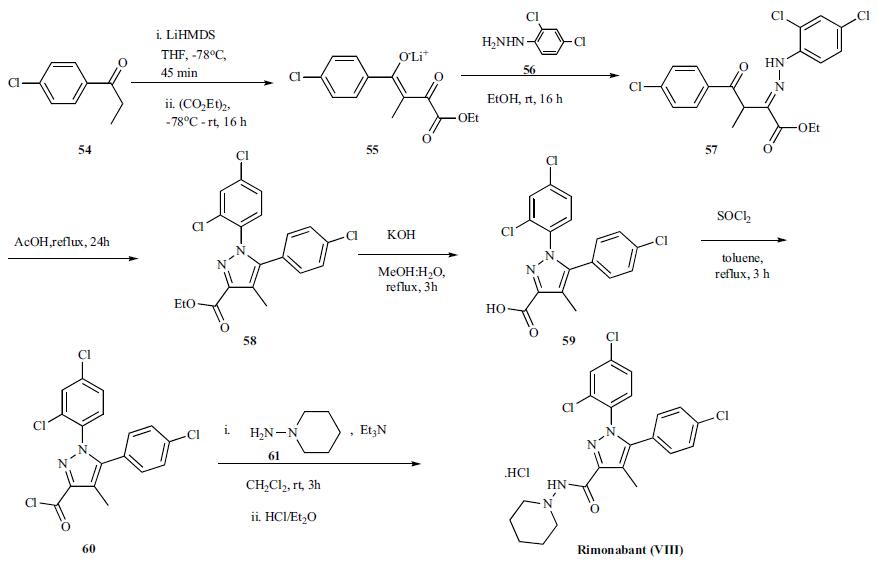
| | 参考文献 | 1) Rinaldi-Carmona et al. (1994) SR141716A, a potent and selective antagonist of the brain cannabinoid receptor; FEBS Lett. 350 240
2) Rinaldi-Carmona et al. (1995) Biochemical and pharmacological characterization of SR141716A, the first potent and selective cannabinoid receptor antagonist; Life Sci. 56 1941 |
|