- Flupentixol Impurity 5
-

- $0.00 / 10mg
-
2025-12-16
- CAS:492-22-8
- Min. Order: 10mg
- Purity: 95%+
- Supply Ability: 100000
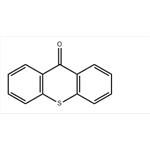
- Thioxanthen-9-one
-
- $1.00 / 1KG
-
2025-12-12
- CAS:492-22-8
- Min. Order: 1KG
- Purity: 95%
- Supply Ability: 600KG
- Thiaxanthone
-

- $10.00 / 1kg
-
2025-05-23
- CAS:492-22-8
- Min. Order: 1kg
- Purity: 99%
- Supply Ability: 20 ton
Related articles - Uses of Thioxanthone
- Thioxanthone is a heterocyclic compound that is a sulfur analog of xanthone.
- Mar 23,2022
|
| | Thioxanthen-9-one Basic information |
| | Thioxanthen-9-one Chemical Properties |
| Melting point | 210-213 °C(lit.) | | Boiling point | 371-373 °C715 mm Hg(lit.) | | density | 1.2247 (rough estimate) | | refractive index | 1.5700 (estimate) | | Fp | 371-373°C/715mm | | storage temp. | 2-8°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | color | Pale Yellow to Light Yellow | | Water Solubility | practically insoluble | | Merck | 14,9369 | | BRN | 140978 | | Stability: | Stable. Incompatible with strong oxidizing agents. | | InChI | 1S/C13H8OS/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H | | InChIKey | YRHRIQCWCFGUEQ-UHFFFAOYSA-N | | SMILES | O=C1c2ccccc2Sc3ccccc13 | | CAS DataBase Reference | 492-22-8(CAS DataBase Reference) | | NIST Chemistry Reference | Thioxanthone(492-22-8) | | EPA Substance Registry System | Thioxanthone (492-22-8) |
| Hazard Codes | Xi | | Risk Statements | 36/37/38 | | Safety Statements | 22-24/25-37/39-26 | | WGK Germany | 3 | | Hazard Note | Irritant | | TSCA | TSCA listed | | HS Code | 29349990 | | Storage Class | 13 - Non Combustible Solids |
| | Thioxanthen-9-one Usage And Synthesis |
| Description | Thioxanthone is a heterocyclic compound that is a sulfur analog of xanthone.
Thioxanthone can be prepared by the reaction of diphenyl sulfide with phosgene in the presence of catalytic aluminium chloride. This synthesis can be seen as a special case of the Friedel-Crafts acylation. The reduction product is thioxanthene.
Thioxanthone dissolves in concentrated sulfuric acid to give a yellow colored liquid with intense green fluorescence. A mixture of the thioxanthone derivatives of 2- and 4-isopropylthioxanthone (ITX) is used in the printing industry. Pharmaceutical drugs that are derivatives of thioxanthone include hycanthone and lucanthone. | | Chemical Properties | slightly yellow crystalline powder | | Uses | Thioxanthen-9-one is a reagent and a starting material for the synthesis of Metixene Hydrochloride. It is also used for highly functional group tolerant and chemoselective oxidation of aromatic or aliphatic sulfides to sulfoxides with hydrogen peroxide. | | Synthesis Reference(s) | Tetrahedron Letters, 35, p. 2195, 1994 DOI: 10.1016/S0040-4039(00)76794-3 | | Reactivity Profile | Thioxanthen-9-one can mediate reactions via triplet energy transfer (EnT), hydrogen atom transfer (HAT) or single electron transfer (SET). Apart from the use in polymerisation chemistry, thioxanthone and its derivatives have been employed in a wide variety of chemical transformations, such as photoisomerisation reactions, photoinduced rearrangements, [2 + 2] photocycloadditions, Paterno–Buchi cycloadditions, photoredox catalysis and cross-coupling reactions leading to C–O, C–N, C–P and other bond formation[1]. | | Purification Methods | It forms yellow needles from CHCl3 or EtOH and sublimes in vacuo. It is soluble in CS2, hot AcOH, and dissolves in conc H2SO4 to give a yellow colour with green fluorescence in VIS light. The sulfone has m 187o (from EtOH), and the hydrazone has m 115o (yellow leaflets from EtOH/*C6H6). The oxime has m 194-196o (from pet ether). [Szmant et al. J Org Chem 18 745 1953, Ullmann et al. Chem Ber 49 2509 1916, NMR: Sharpless et al. Org Magn Res 6 115 1974, Beilstein 17 H 357, 17 I 191, 17 III/IV 5302, 17/10 V 437.] | | Structure and conformation | Thioxanthen-9-one (TX) is a aromatic ketone. In comparison with other aromatic ketones, TX has a high triplet energy and a relatively long triplet lifetime, while it has the ability to participate successfully in merger reactions with metal complexes. The conformation of TX is not fixed, but it interconverts from a planar (C2v) to a non-planar conformation. In the case of TX, the intersystem crossing of an electron normally occurs from a non-bonding orbital (n) to an anti-bonding pi orbital (π*) (nπ* configuration) and occurs 103 times faster than in the case of migration from the same type of molecular orbital (ππ* migration).Theoretical studies support that TX's low lying triplet state is close enough to the singlet state, favoring in this way internal conversion (IC) and ISC upon photoexcitation. Due to its high triplet energy, TX is an efficient sensitizer for oxygen, leading to singlet oxygen, since the excitation of oxygen requires around 23 kcal mol-1[1].
| | References | [1] Nikitas N, et al. Thioxanthone: a powerful photocatalyst for organic reactions. Organic & Biomolecular Chemistry, 2021; 19: 5237-5253. |
| | Thioxanthen-9-one Preparation Products And Raw materials |
|