| Company Name: |
ChemeGen(Shanghai) Biotechnology Co.,Ltd.
|
| Tel: |
18818260767 |
| Email: |
sales@chemegen.com |
| Products Intro: |
Product Name:Zamifenacin
CAS:127308-82-1
Purity:98% Package:10 mg;50 mg;100 mg;500 mg;1 g;5 g;10 g
|
| Company Name: |
Beijing Jin Ming Biotechnology Co., Ltd.
|
| Tel: |
010-60605840 15801484223 |
| Email: |
psaitong@jm-bio.com |
| Products Intro: |
Product Name:Zamifenacin
CAS:127308-82-1
Purity:98% Package:5mg;10mg;25mg;50mg;100mg
|
|
| | ZAMIFENACIN Basic information |
| Product Name: | ZAMIFENACIN | | Synonyms: | (R)-3-(Benzhydryloxy)-1-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)piperidine;Piperidine, 1-[2-(1,3-benzodioxol-5-yl)ethyl]-3-(diphenylmethoxy)-, (3R)-;UK-76654 | | CAS: | 127308-82-1 | | MF: | C27H29NO3 | | MW: | 415.52 | | EINECS: | | | Product Categories: | | | Mol File: | 127308-82-1.mol | 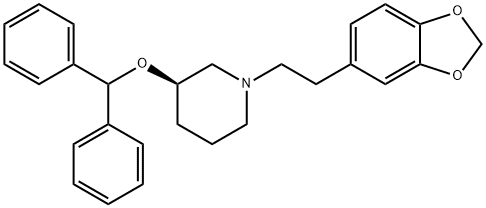 |
| | ZAMIFENACIN Chemical Properties |
| storage temp. | Store at -20°C |
| | ZAMIFENACIN Usage And Synthesis |
| Originator | Zamifenacin,Sumitomo Industries | | Definition | ChEBI: (3R)-1-[2-(1,3-benzodioxol-5-yl)ethyl]-3-(diphenylmethyl)oxypiperidine is a diarylmethane. | | Manufacturing Process | (3R)-Diphenylmethoxy-1-(3,4-methylenedioxyphenethyl)piperidine:
A mixture of (3R)-diphenylmethoxypiperidine (2.67 g), 3,4-
methylenedioxyphenethyl bromide (2.29 g), sodium carbonate (2.10 g) and
sodium iodide (0.25 g) in acetonitrile (50 ml) was heated under reflux for 68
hours, diluted with ethyl acetate and water, and the layers were separated.
The organic layer was washed with water, dried over magnesium sulfate and
evaporated. The residue was purified by chromatography on silica (50 g) using
methylene chloride containing 0-5% methanol as the eluant. The title
compound (zamifenacin) was obtained as a colourless solid after
recrystallisation from hexane (1.25 g, 78%), MP: 52°-55°C, [α] D 25 = +22.5°
(c 1.5 in ethanol).
The starting compounds were prepared next way:
1. (3R)-Diphenylmethoxypiperidine:
A solution of (3R)-hydroxypiperidinium (1S)-camphor-10-sulphonate prepared
from (3R,S)-hydroxypiperidine by the method of B. Ringdahl, U. F. W.
Ohnsorge and J. C. Craig, [J. Chem. Soc. Perkin II, (1981), 697],
[α] D 25 =+23.1° (c 1.5 in 50% aqueous ethanol; (1 mole-equivalent),
benzhydrol (1mole-equivalent) and p-toluenesulphonic acid monohydrate (1
mole-equivalent) in toluene (600 ml) was heated under reflux for four hours
using a Dean-Stark apparatus to remove the water formed. The mixture was
then partitioned between 2 M aqueous sodium hydroxide solution and ethyl
acetate and the organic layer was washed with water and evaporated. The
residue was partitioned between ether and 10% aqueous citric acid and the
acidic layer was washed with ether, basified with excess solid sodium
carbonate and extracted into ether. The organic layer was washed with water,
dried over magnesium sulfate and evaporated to give the title compound a
colourless oil (2.7 g, 50%), [α] D 25 =+3.3° (c 1.5 in ethanol), which was
characterized by its 1 H-NMR spectrum.
2. 3,4-Methylenedioxyphenethyl alcohol:
3,4-Methylenedioxyphenylacetic acid (18.0 g) was added portion wise over 30
minutes to a stirred, ice-cooled suspension of lithium aluminium hydride (4.0
g) in ether (400 ml) and the mixture was stirred at temperature for two
hours, quenched by the cautious addition of saturated aqueous ammonium
chloride solution and filtered. The filtrate was washed with 10% aqueous
sodium carbonate solution, dried over magnesium sulfate and evaporated to
give the title compound as a pale yellow oil (15.01 g, 90%), which was
characterized by its 1 H-NMR spectrum.
3. 3,4-Methylenedioxyphenethyl bromide:
A solution of phosphorus tribromide (8.1 g) in carbon tetrachloride (50 ml)
was added dropwise over 30 minutes to a stirred solution of 3,4-
methylenedioxyphenethyl alcohol (15.0 g) in carbon tetrachloride (200 ml)
and the mixture was heated under reflux for 3 hours, washed sequentially
with water (twice), 5 M aqueous sodium hydroxide solution and water, dried over magnesium sulfate and evaporated. The residue was purified by
chromatography on silica (100 g) using carbon tetrachloride as the eluant.
Appropriate fractions were combined and evaporated to give the 3,4-
methylenedioxyphenethyl bromide as a pale yellow oil (8.3 g, 40%), which
was characterized by its 1 H-NMR spectrum.
Zamifenacin may be also synthesized from L-proline methyl ester in 4 steps
an overall yield of 20% by using a ring enlargement of L-proline derivative. | | Therapeutic Function | Antimuscarinic |
| | ZAMIFENACIN Preparation Products And Raw materials |
|