| | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON Basic information |
| Product Name: | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON | | Synonyms: | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON;Pentazocine Hydrochloride {D.D.};pentazocine hydrochloride--dea schedule iv item;(2alpha,6alpha,11R*)-1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methylbut-2-enyl)-2,6-methano-3-benzazocin-8-ol hydrochloride;2,6-Methano-3-benzazocin-8-ol, 1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-, hydrochloride, (2R,6R,11R)-rel-;Pentzocine hydrochloride;D02227;Pentazocine hydrochloride (jan/usp) | | CAS: | 64024-15-3 | | MF: | C19H28ClNO | | MW: | 321.89 | | EINECS: | 264-612-1 | | Product Categories: | | | Mol File: | 64024-15-3.mol | 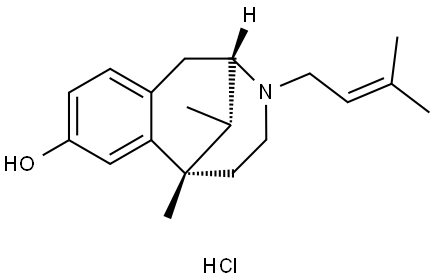 |
| | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON Chemical Properties |
| solubility | Sparingly soluble in water, soluble in ethanol (96 per cent) and sparingly soluble in methylene chloride. |
| | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON Usage And Synthesis |
| Chemical Properties | A white or almost white powder | | Originator | Pentazocine,Mallinckrodt Inc. | | Uses | Opioid receptor agonist; analgesic. | | Manufacturing Process | A solution of 3,4-dimethylpyridine was added to a methyliodid. Then to the resulting solution containing 1,3,4-trimethylpyridinium iodide the 4methoxybenzylmagnesium chloride was added. After reaction process the 1,3,4-trimethyl-2-(4-methoxy-benzyl)-pyridine was obtained.
To the solution of 1,3,4-trimethyl-2-(4-methoxy-benzyl)-pyridine was reduced by hydrogen over 10% palladium-on-charcoal, and when reduction was complete, the catalyst was removed by filtration and the filtrate taken to dryness. The residue was recrystallized to give 2-(4-methoxybenzyl)-1,3,4trimethyl-1,2,5,6-tetrahydropyridine.
To the 2-(4-methoxybenzyl)-1,3,4-trimethyl-1,2,5,6-tetrahydropyridine the solution of hydrobromic acid was added and heated under reflux.The product obtained was recrystallized and yield N-methyl-1,2,3,4,5,6-hexahydro-6,11dimethyl-8-hydroxy-2,6-methano-3-benzazocine (2'-hydroxy-2,5,9trimethylbenzo-6-morphen), which then was demethylated by bromcyan (BrCN). As a result the racemic cis-1,2,3,4,5,6-hexahydro-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine was obtained (that is a. 2'-hydroxy-5,9dimethyl-6,7-benzomorphen).
A mixture of 8.7 g racemic cis-1,2,3,4,5,6-hexahydro-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine, 6.0 g of 1-bromo-3-methyl-2-butene, 5.0 g of sodium bicarbonate, and 125 ml of N,N-dimethylformamide was stirred and refluxed for approximately 4.5 hours. The reaction mixture was then filtered, and the solid on the filter was washed with ethanol. The filtrate and the wash liquor were combined, concentrated under reduced pressure, and then extracted with chloroform. The chloroform extract was concentrated under reduced pressure to yield a syrup which weighed 15.8 g. This syrup was dissolved in 120 ml of diethyl ether and the resulting solution was filtered to remove approximately 0.5 g of a brown amorphous solid. The filtrate was extracted with a mixture of 5 ml of concentrated hydrochloric acid and 20 ml of water. To the extract there was added 5 ml of concentrated ammonium hydroxide solution and ice. A pale tan syrup separated from solution and after stirring, this syrup solidified. The resulting pale tan solid was collected and dried; it weighed 10.6 g. After two recrystallizations from a mixture of methyl alcohol and water, with charcoaling, the 1,2,3,4,5,6-hexahydro-3-(3-methyl-2butenyl)-6,11-dimethyl-8-hydroxy-2,6-methano-3-benzazocine weighed 8.2 g and melted at 145°-147°C.
The1,2,3,4,5,6-hexahydro-3-(3-methyl-2-butenyl)-6,11-dimethyl-8-hydroxy2,6-methano-3-benzazocinewas soluble in a mixture of 0.35 ml of 2 N hydrochloric acid and 0.15 ml of water to the extent of 10%, the pH of the 1% solution being 2.80; and when the pH of the 1% solution was gradually raised by addition of 10 N sodium hydroxide solution, a precipitate formed at pH 5.4. The 1,2,3,4,5,6-hexahydro-3-(3-methyl-2-butenyl)-6,11-dimethyl-8hydroxy-2,6-methano-3-benzazocine hydrochloride melted at 245°-247°C, dec. | | Brand name | Talwin (Sanofi Aventis). | | Therapeutic Function | Analgesic |
| | (+)-PENTAZOCINE MIXED AGONIST/ANTAGON Preparation Products And Raw materials |
|