| | bezitramide Basic information |
| Product Name: | bezitramide | | Synonyms: | bezitramide;1-[1-(3-Cyano-3,3-diphenylpropyl)-4-piperidinyl]-1,3-dihydro-3-(1-oxopropyl)-2H-benzimidazol-2-one;R 4845;1-Piperidinebutanenitrile, 4-[2,3-dihydro-2-oxo-3-(1-oxopropyl)-1H-benzimidazol-1-yl]-α,α-diphenyl-;FLKWNFFCSSJANB-UHFFFAOYSA-N | | CAS: | 15301-48-1 | | MF: | C31H32N4O2 | | MW: | 492.61 | | EINECS: | 239-335-4 | | Product Categories: | | | Mol File: | 15301-48-1.mol | 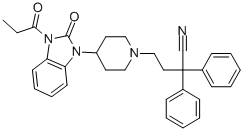 |
| | bezitramide Chemical Properties |
| Melting point | 145-149°; mp 124.5-126° | | Boiling point | 586.35°C (rough estimate) | | density | 1.1470 (rough estimate) | | refractive index | 1.6000 (estimate) | | pka | 8.21±0.10(Predicted) | | color | White, crystalline powder |
| Toxicity | LD50 orally in mice, rats: 2101, 141 mg/kg (Janssen) |
| | bezitramide Usage And Synthesis |
| Originator | Bezitramide,ZYF Pharm Chemical | | Uses | Bezitramide is a narcotic analgesic agonist with modified pipiridine N-substituents whose binding affinity to the ORL1 and N-methyl-D-aspartate (NMDA) receptors is of research interest. | | Definition | ChEBI: Bezitramide is a nitrile. | | Manufacturing Process | A mixture of 6.9 parts 4-bromo-2,2-diphenylbutyronitrile, 5 parts 4-(2-oxo-1-
benzimidazolinyl)piperidine, 7.3 parts sodium carbonate, a few crystals of
potassium iodide in 160 parts 4-methyl-2-pentanone is stirred and refluxed
for 12 hours. After cooling the reaction mixture, 100 parts water is added.
The aqueous layer is separated and extracted with 4-methyl-2-pentanone. The
combined organic layer are dried over MgSO4 and evaporated. The oily
residue is dissolved in a mixture of 24 parts diisopropylether and 24 parts
isopropanol. After cooling overnight to -20°C, 5.3 parts product are obtained.
This crop is boiled in 72 parts 4-methyl-2-pentanone and cooled to 0°C,
yielding 1-(3-cyano-3,3-diphenylpropyl)-4-(2-oxo-1-benzimidazolinyl)
piperidine, melting point 225-226°C, as a grey-white amorphous powder.
A mixture of 5 parts 1-(3-cyano-3,3-diphenylpropyl)-4-(2-oxo-1-
benzimidazolinyl)piperidine, 7.5 parts propionic acid anhydride and 80 parts
benzene is stirred and refluxed for 16 hours. After cooling, the reaction
mixture is washed twice with 100 parts water. The aqueous layer is dried over
potassium carbonate, filtered and evaporated. The residue is recrystallized
from 60 parts of ether, yielding 4 parts crude 1-(3-cyano-3,3-diphenylpropyl)-
4-(2-oxo-3-propionyl-1-benzimidazolinyl)piperidine. This crop is recrystallized
from 20 parts m-methyl-2-pentanone 4-ethyl-2-pentanone, yielding 1-(3-cyano-3,3-diphenylpropyl)-4-(2-oxo-3-propionyl-1-benzimidazolinyl)piperidine
with melting point: 124.5-126°C as a pale yellow amorphous powder. | | Therapeutic Function | Narcotic analgesic, Antitussive | | Safety Profile | Poison by ingestion. Caution: Maybe habit forming. This is a controlled substance (opiate)listed in the U.S. Code of Federal Regulations, Title 21Part 1308.12 (1985). When heated to decomposition itemits toxic fumes of NOx and CN-. |
| | bezitramide Preparation Products And Raw materials |
|