|
|
| | MAGNESIUM BISULFITE Basic information |
| Product Name: | MAGNESIUM BISULFITE | | Synonyms: | sulfurousacid,magnesiumsalt(2:1);Magnesium acid sulfite;MAGNESIUM BISULFITE;MAGNESIUM HYDROGEN SULFITE;Magnesium bisulfite solution;magnesium dihydrogen disulphite;MAGNESIUM BISULFITE, APPROX. 30 WT. % SO LUTION IN WATER;Bis(sulfinooxy)magnesium | | CAS: | 13774-25-9 | | MF: | H4MgO3S | | MW: | 108.39 | | EINECS: | 237-403-8 | | Product Categories: | Inorganics | | Mol File: | 13774-25-9.mol | 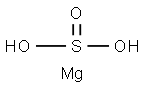 |
| | MAGNESIUM BISULFITE Chemical Properties |
| Hazard Codes | C | | Risk Statements | 34 | | Safety Statements | 23-26-27-36/37/39-45 | | RIDADR | UN 2693 8/PG 3 | | HazardClass | 8 | | PackingGroup | III |
| | MAGNESIUM BISULFITE Usage And Synthesis |
| General Description | An aqueous solution of magnesium hydrogen sulfite. Corrosive. | | Air & Water Reactions | An aqueous solution. | | Reactivity Profile | MAGNESIUM BISULFITE generates gaseous toxic sulfur dioxide in contact with oxidizing acids and nonoxidizing acids. Reacts with oxidizing agents to generate heat and products that may be flammable, combustible, or otherwise reactive. Can react explosively with strong oxidizing agents. | | Health Hazard | TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution. | | Fire Hazard | Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. |
| | MAGNESIUM BISULFITE Preparation Products And Raw materials |
|