|
|
| | 2-Amino-1,3,4-thiadiazole Basic information |
| Product Name: | 2-Amino-1,3,4-thiadiazole | | Synonyms: | 5-nitro-1,3,4-thiadiazol-2-aMine;2-AMino-1,3,4-thiadiazole, 97% 10GR;5-AMino-1,3,4-thiadiazole;2-AMino-1,3,4-thiadiazole 97%;1,3,4-Thiadiazole, 2-amino-;1,3,4-Thiadiazole-2-Amine;2-Amino-1-thia-3,4-diazole;2-Aminothiadiazole | | CAS: | 4005-51-0 | | MF: | C2H3N3S | | MW: | 101.13 | | EINECS: | 223-657-7 | | Product Categories: | Intermediates & Fine Chemicals;Pharmaceuticals;Sulfur & Selenium Compounds;Oxadiazoles & Thiadiazoles;VARIOUSAMINE;Amines;Oxadiazoles & Thiadiazoles;Thiazole;API intermediates;Aromatic;Heterocycles;bc0001 | | Mol File: | 4005-51-0.mol | 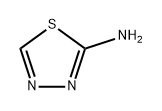 |
| | 2-Amino-1,3,4-thiadiazole Chemical Properties |
| Melting point | 188-191 °C (dec.) (lit.) | | Boiling point | 238.9±23.0 °C(Predicted) | | density | 1.385 (estimate) | | refractive index | 1.5800 (estimate) | | storage temp. | Keep in dark place,Sealed in dry,Room Temperature | | solubility | water: soluble25mg/mL, clear, colorless | | pka | pKa 3.2 (Uncertain) | | form | Crystals or Crystalline Powder | | color | White to light yellow | | Water Solubility | It is soluble in water, hot methanol- very faint turbidity. | | BRN | 107135 | | InChIKey | QUKGLNCXGVWCJX-UHFFFAOYSA-N | | CAS DataBase Reference | 4005-51-0(CAS DataBase Reference) | | NIST Chemistry Reference | 2-Amino-1,3,4-thiadiazole(4005-51-0) |
| Hazard Codes | Xn,Xi | | Risk Statements | 68-63-36/37/38-20/21/22-22 | | Safety Statements | 36/37/39-26-36 | | RIDADR | 2811 | | WGK Germany | 3 | | RTECS | XI3000000 | | Hazard Note | Harmful | | HazardClass | 6.1 | | PackingGroup | III | | HS Code | 29349990 | | Toxicity | LD50 oral in rat: 253mg/kg |
| | 2-Amino-1,3,4-thiadiazole Usage And Synthesis |
| Chemical Properties | white to light yellow crystal powde | | Uses | A thiadiazole derivative with fungacidal properties. An antitumor agent. | | Uses | 2-Amino-1,3,4-thiadiazole, is used as an intermediate for ceftraoline. | | General Description | 2-Amino-1,3,4-thiadiazole (donor) form charge transfer complexes with 2,3-dichloro-5,6-dicyano-p-benzoquinone, p-chloranil, o-chloranil, p-bromanil and chloranilic acid (acceptors). Effects of 2-amino-1,3,4-thiadiazole [aminothiadiazole (NSC 4728)] on purine and pyrimidine ribonucleotide pools of L1210 ascites cells in vivo has been reported. | | Safety Profile | Poison by subcutaneous andintraperitoneal routes. An experimental teratogen. Otherexperimental reproductive effects. When heated todecomposition it emits very toxic fumes of NOx and SOx. | | Synthesis | General procedure for the synthesis of 2-amino-1,3,4-thiadiazole from formic acid and aminothiourea: 5.00 g of aminothiourea was accurately weighed and placed in a 50 mL three-necked flask fitted with a stirring magnet, and 5.00 mL of formic acid was added at once. The reaction was stirred under the condition of cooling in an ice bath, and after the formation of a slurry, 6.00 mL of concentrated hydrochloric acid was added slowly and dropwise. After the dropwise addition, the reaction mixture was transferred to an oil bath preheated to 107 °C with stirring turned on and the reaction was carried out for 4.5 to 5.0 h. During this time, the reaction progress was monitored by thin-layer chromatography (TLC), and the reaction was stopped when the raw material point disappeared. After the reaction solution was cooled to room temperature, the pH was adjusted with concentrated ammonia to 8-9. The mixture was placed in a refrigerator overnight, white crystals were precipitated, filtered, washed three times with ice water, and finally recrystallized with distilled water to give 4.96 g of white crystal product in about 90% yield. The melting point of the product was 198 to 201 °C. The resulting product was confirmed to be 2-amino-1,3,4-thiadiazole by infrared spectroscopy (IR), nuclear magnetic resonance (NMR) and mass spectrometry (MS) analysis. | | References | [1] Nature Communications, 2017, vol. 8, # 1,
[2] Journal of Chemical Research, 2005, # 5, p. 341 - 343
[3] Journal of the Chemical Society of Pakistan, 2015, vol. 37, # 1, p. 115 - 121
[4] Patent: CN106117268, 2016, A. Location in patent: Paragraph 0030; 0031
[5] Patent: CN106167502, 2016, A. Location in patent: Paragraph 0032; 0033 |
| | 2-Amino-1,3,4-thiadiazole Preparation Products And Raw materials |
| Raw materials | Hydrazinecarboxamide, 2-formyl--->Methanimidic acid ethyl ester-->Methanone, (5-amino-1,3,4-thiadiazol-2-yl)phenyl--->Benzamide, N-1,3,4-thiadiazol-2-yl--->Formamide, N-1,3,4-thiadiazol-2-yl--->Methanimidamide,N,N'-di-1,3,4-thiadiazol-2-yl--->CL 19506 7-5526-->1-formylthiosemicarbazide-->thiosemicarbazide-->Formic acid | | Preparation Products | 2-AMINO-5-BROMO-[1,3,4]THIADIAZOLE-->2-Mercapto-1,3,4-thiadiazol |
|