| 外観 | 白色~ほとんど白色粉末~結晶 |
| 解説 | ピラジナミド.ピラジンカルボン酸メチルのアンモニア分解で得られる.白色の結晶.融点188~193 ℃.λmax 269 nm.pKa 0.5.水,メタノールに難溶.肺結核治療薬として,イソニコチン酸ヒドラジドと併用して使用される. |
| 効能 | 抗結核薬 |
| 商品名 | ピラマイド (アルフレッサファーマ) |
| 説明 | Pyrazinamide was synthesized in 1952, and it is the nitrogen-analog of nicotinamide. It
exhibits hepatotoxicity. Synonyms of this drug are dexambutol, miambutol, esnbutol, ebu�tol, and others. |
| 化学的特性 | Crystalline Solid |
| 使用 | Pyrazinamide is used therapeutically as an antitubercular agent. Pyrazinamide is used to form polymeric copper complexes, create pyrazine carboxamide scaffolds useful as FXs inhibitors, and as a component of mycobacteria identification kits. It is used to study liver toxicity prevention and mechanisms of resistance . |
| 使用 | An antibacterial agent used to study liver toxicity prevention |
| 使用 | Antibacterial (tuberculostatic) |
| 適応症 | Pyrazinamide is a synthetic analogue of nicotinamide.
Its exact mechanism of action is not known, although
its target appears to be the mycobacterial fatty acid synthetase involved in mycolic acid biosynthesis.
Pyrazinamide requires an acidic environment, such as
that found in the phagolysosomes, to express its tuberculocidal
activity. Thus, pyrazinamide is highly effective
on intracellular mycobacteria. The mycobacterial enzyme
pyrazinamidase converts pyrazinamide to pyrazinoic
acid, the active form of the drug.A mutation in the
gene (pncA) that encodes pyrazinamidase is responsible
for drug resistance; resistance can be delayed
through the use of drug combination therapy. |
| 定義 | ChEBI: Pyrazinecarboxamide is a monocarboxylic acid amide resulting from the formal condensation of the carboxy group of pyrazinoic acid (pyrazine-2-carboxylic acid) with ammonia. A prodrug for pyrazinoic acid, pyrazinecarboxamide is used as part of multidrug regimens for the treatment of tuberculosis. It has a role as an antitubercular agent and a prodrug. It is a member of pyrazines, a N-acylammonia and a monocarboxylic acid amide. |
| 抗菌性 | It is principally active against actively metabolizing intracellular
bacilli and those in acidic, anoxic inflammatory lesions.
Activity against M. tuberculosis is highly pH dependent: at pH
5.6 the MIC is 8–16 mg/L, but it is almost inactive at neutral
pH. Other mycobacterial species, including M. bovis, are resistant.
Activity requires conversion to pyrazinoic acid by the
mycobacterial enzyme pyrazinamidase, encoded for by the
pncA gene, which is present in M. tuberculosis but not M. bovis.
A few resistant strains lack mutations in pncA, indicating alternative
mechanisms for resistance, including defects in transportation
of the agent into the bacterial cell. |
| 獲得抵抗性 | Drug resistance is uncommon and cross-resistance to other
antituberculosis agents does not occur. Susceptibility testing
is technically demanding as it requires very careful control of
the pH of the medium, but molecular methods for detection
of resistance-conferring mutations are available. |
| 一般的な説明 | Pyrazinecarboxamide (PZA) occurs as a white crystalline powder that is sparingly soluble in water and slightly soluble in polar organic solvents. Its antitubercular properties were discovered as a result of an investigation of heterocyclic analogs of nicotinic acid, with which it is isosteric. Pyrazinamide has recently been elevated to first-line status in short-term tuberculosis treatment regimens because of its tuberculocidal activity and comparatively low short-term toxicity. Since pyrazinamide is not active against metabolically inactive tubercle bacilli, it is not considered suitable for long-term therapy. Potential hepatotoxicity also obviates long-term use of the drug. Pyrazinamide is maximally effective in the low pH environment that exists in macrophages (monocytes). Evidence suggests bioactivation of pyrazinamide to pyrazinoic acid by an amidase present in mycobacteria. |
| 一般的な説明 | White powder. Sublimes from 318°F. |
| 空気と水の反応 | Water soluble. |
| 反応プロフィール | Pyrazinamide is a carbamate ester. Incompatible with strong acids and bases, and especially incompatible with strong reducing agents such as hydrides. May react with active metals or nitrides to produce flammable gaseous hydrogen. Incompatible with strongly oxidizing acids, peroxides, and hydroperoxides. |
| 応用例(製薬) | Like isoniazid, pyrazinamide is a synthetic nicotinamide analog,
although its mode of action is quite distinct. |
| Biochem/physiol Actions | The active moiety of pyrazinamide is pyrazinoic acid (POA). POA is thought to disrupt membrane energetics and inhibit membrane transport function at acid pH in Mycobacterium tuberculosis. Iron enhances the antituberculous activity of pyrazinamide . Pyrazinamide and its analogs have been shown to inhibit the activity of purified FAS I. |
| 薬物動態学 | Oral absorption: >90%
Cmax 20–22 mg/kg oral: 10–50 mg/L after 2 h
Plasma half-life: c. 9 h
Plasma protein binding: c. 50%
It readily crosses the blood–brain barrier, achieving CSF
concentrations similar to plasma levels. It is metabolized to
pyrazinoic acid in the liver and oxidized to inactive metabolites,
which are excreted in the urine, although about 70% of
an oral dose is excreted unchanged. |
| 薬理学 | Pyrazinamide is well absorbed from the GI tract and
is widely distributed throughout the body. It penetrates
tissues, macrophages, and tuberculous cavities and has
excellent activity on the intracellular organisms; its
plasma half-life is 9 to 10 hours in patients with normal
renal function. The drug and its metabolites are excreted
primarily by renal glomerular filtration. |
| 臨床応用 | Tuberculosis (a component of the early, intensive phase of short-course
therapy) |
| 臨床応用 | Pyrazinamide is an essential component of the multidrug
short-term therapy of tuberculosis. In combination
with isoniazid and rifampin, it is active against the
intracellular organisms that may cause relapse. |
| 副作用 | Hepatotoxicity is the major concern in 15% of pyrazinamide
recipients. It also can inhibit excretion of urates,
resulting in hyperuricemia. Nearly all patients taking
pyrazinamide develop hyperuricemia and possibly acute
gouty arthritis. Other adverse effects include nausea,
vomiting, anorexia, drug fever, and malaise. Pyrazinamide
is not recommended for use during pregnancy. |
| 副作用 | It is usually well tolerated. Moderate elevations of serum
transaminases occur early in treatment. Severe hepatotoxicity
is uncommon with standard dosage, except in patients with
pre-existing liver disease.
Its principal metabolite, pyrazinoic acid, inhibits renal
excretion of uric acid, but gout is extremely rare. An unrelated
arthralgia, notably of the shoulders and responsive to
analgesics, also occurs.
Other side effects include anorexia, nausea, mild flushing
of the skin and photosensitization. |
| 合成 | Pyrazinamide, pyrazincarboxamide (34.1.11), is synthesized from quinox�aline (34.1.7) by reacting o-phenylendiamine with glyoxal. Oxidation of this compound
with sodium permanganate gives pyrazin-2,3-dicarboxylic acid (34.1.8). Decarboxylation
of the resulting product by heating gives pyrazin-2-carboxylic acid (34.1.9). Esterifying
the resulting acid with methanol in the presence of hydrogen chloride and further reaction
of this ester (34.1.10) with ammonia gives pyrazinamide. 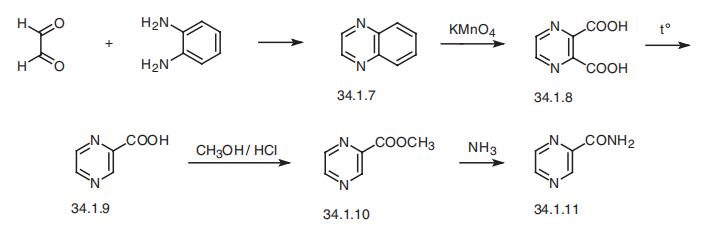
Pyrazinamide was synthesized in 1952, and it is the nitrogen-analog of nicotinamide. It
exhibits hepatotoxicity. Synonyms of this drug are dexambutol, miambutol, esnbutol, ebu�tol, and others. |
| 薬物相互作用 | Potentially hazardous interactions with other drugs
Ciclosporin: on limited evidence, pyrazinamide
appears to reduce ciclosporin levels. |
| 代謝 | Pyrazinamide is metabolised mainly in the liver by
hydrolysis to the major active metabolite pyrazinoic acid,
which is subsequently hydroxylated to the major excretory
product 5-hydroxypyrazinoic acid.
It is excreted via the kidneys mainly by glomerular
filtration. About 70% of a dose appears in the urine
within 24 hours mainly as metabolites. |
| 純化方法 | The amide crystallises from water, EtOH or 1:1 hexane/EtOH in four modifications viz �-form, �-form, -form and ��form. [R. & S.rum Acta Cryst 28B 1677 1972, Beilstein 25 III/IV 772.] |