|
|
| | LOFEPRAMINE Basic information |
| Product Name: | LOFEPRAMINE | | Synonyms: | LOFEPRAMINE;Gamanil;1-(4-Chlorophenyl)-2-[[3-(10,11-dihydro-5H-dibenz[b,f]azepin-5-yl)propyl]methylamino]ethanone;4'-Chloro-2-[[3-(10,11-dihydro-5H-dibenz[b,f]azepin-5-yl)propyl-methylamino]acetophenone;Amplit;Leo 640;Lopramine;1-(4-Chlorophenyl)-2-[[3-(10,11-dihydro-5H-dibenz(Z)[b,f]azepin-5-yl)propyl]methylamino]ethanone | | CAS: | 23047-25-8 | | MF: | C26H27ClN2O | | MW: | 418.96 | | EINECS: | 245-396-8 | | Product Categories: | Aromatics;Heterocycles;Intermediates & Fine Chemicals;Pharmaceuticals | | Mol File: | 23047-25-8.mol | 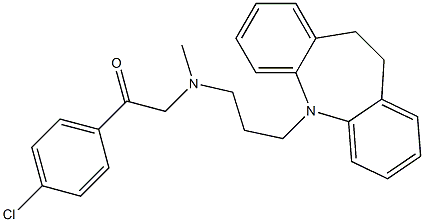 |
| | LOFEPRAMINE Chemical Properties |
| Melting point | 103-105°C | | vapor pressure | 0Pa at 25℃ | | storage temp. | Store at +4°C | | solubility | Chloroform (Slightly), Methanol (Slightly) | | form | Solid | | color | Light Yellow to Light Beige | | Water Solubility | 2.04mg/L at 25℃ | | Stability: | Unstable in Solution |
| | LOFEPRAMINE Usage And Synthesis |
| Description | Lofepramine is a first generation tricyclic antidepressant that is extensively metabolized to desipramine. It potently inhibits serotonin and norepinephrine transporters (Kds = 70 and 5.4 nM, respectively) and less potently antagonizes serotonin, histamine, and muscarinic receptors. | | Chemical Properties | Light Yellow Solid | | Originator | Gamonil,E. Merck | | Uses | Psychotropic drug related to imipramine. Antidepressant. | | Definition | ChEBI: Lofepramine is a dibenzoazepine, a member of monochlorobenzenes, a tertiary amino compound and an aromatic ketone. It has a role as an antidepressant. | | Manufacturing Process | 9.8 parts of 10,11-dihydro-5H-dibenzo[b,f]azepine are dissolved in 10 parts of
dry toluene and 3.1 parts of sodium amide are added and the mixture is
refluxed and stirred for four hours. A solution of 13.5 parts of 1-(4-
chlorophenyl)-2-[(3-chloropropyl)methylamino]ethanone in 20 parts of dry
toluene is added dropwise and the mixture is stirred and refluxed for eight
hours.
After cooling to room temperature water is carefully added to the reaction
mixture and the toluene solution is extracted with water to which hydrochloric
acid is added so that the aqueous phase obtains the pH-value of 5. The
aqueous extract is discarded and the toluene phase is evaporated to dryness
in vacuum. The residue is dissolved in 50 parts of methanol. Hydrogen gas is
introduced to give the crystalline hydrochloride 1-(4-chlorophenyl)-2-((3-
(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)propyl)methylamino)ethanone;
MP: 154°-156°C. The hydrochloride may be removed by adding an equivalent
of any base (triethyl amine, sodium hydroxide and so on). | | Therapeutic Function | Antidepressant | | Biological Activity | Serotonin and noradrenalin re-uptake inhibitor (SNRI) that is metabolized to desipramine. Produces inhibition of liver tryptophan pyrollase activity in vitro and displays antidepressant properties in vivo . | | Mechanism of action | Lofepramine differs from imipramine by the attachment of a p-chlorophenacyl moiety to the N-aminopropyl
side chain. This change confers enhanced lipophilicity and the potential of more rapid
distribution into the CNS with greater in vitro affinity and selectivity for NET. Its mechanism of antidepressant
action is attributed to its rapid metabolism to the secondary amine metabolite, desipramine, which selectively
inhibits the neuronal uptake of NE. |
| | LOFEPRAMINE Preparation Products And Raw materials |
|