2-(Di-tert-butylphosphino)-1-phenylindole manufacturers
|
| | 2-(Di-tert-butylphosphino)-1-phenylindole Basic information | | Reaction |
| | 2-(Di-tert-butylphosphino)-1-phenylindole Chemical Properties |
| Melting point | 90-92℃ (methanol ) | | Boiling point | 455.4±27.0 °C(Predicted) | | storage temp. | Refrigerator, under inert atmosphere | | solubility | Acetonitrile (Slightly), Benzene (Slightly), Chloroform (Slightly) | | form | Powder | | color | white to yellow | | Sensitive | air sensitive | | BRN | 9924511 |
| WGK Germany | 3 | | HS Code | 29339900 |
| | 2-(Di-tert-butylphosphino)-1-phenylindole Usage And Synthesis |
| Reaction |
- Useful ligand for the Pd-catalyzed amination reaction.
- Ligand used for the Pd-catalyzed arylation of phenols.
- Useful ligand for the Suzuki-Miyaura coupling.
- Ligand used for the Sonagashira reaction of aryl bromides.
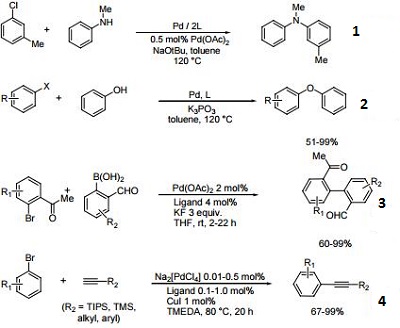
| | Chemical Properties | White to yellow powder | | Uses | N-Phenyl-2-(di-tert-butylphosphino)indole is a catalyst for the selective monoarylation of ammonia with different aryl bromides and chlorides. | | General Description | sold in collaboration with Solvias AG | | reaction suitability | reaction type: Asymmetric synthesis
reagent type: ligand
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reagent type: ligand
reaction type: Sonogashira Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling |
| | 2-(Di-tert-butylphosphino)-1-phenylindole Preparation Products And Raw materials |
|